
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:HC=CH
Reagents available
а. НСI
е. Нэ, Рd
i. H2 / Lindlar's catalyst
b. HBr
OsO4 then NaHSO3
j. Na / NH3
с. Вr2
g. O3 then (CH3)2S
k. (sia)2 BH then H2 O2, NaOH
d. Cl2
h. 2 equivalents of NaNH2 1. 1 equivalent of NaNH2
The above synthesis scheme was designed using the Organic Chemistry Roadmaps in the appendix of your textbook.
In this scheme, identify the reagents necessary to accomplish the following steps in the production of (3R,4S)-3,4-dibromohexane from acetylene:
Step 4:
Step 1:
Step 3:
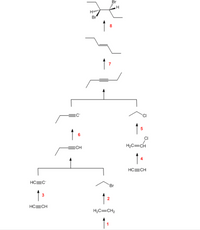
Transcribed Image Text:Br
Hu
Br
7
CI
ECH
H,C=CH
4
HC=CH
HC=C
Br
HC=CH
H2C=CH2
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Name the following compounds. OH b. НО, OH а. с. F. NO2 d. HO Br 2. Provide the missing reagent(s) and major product(s) for each of the following reactions. а. Br AIBR3 b. OH HBr с. OH H,PO4 (conc.) A d. Но AICI,arrow_forwardPredict major products for the following reactions g. h. H2SO4 d. e. NaOH A 1. .CI 1. Li(tBu),AIH من 2. H₂O+ 1. LiAlH4 2. H₂O+ 1. DIBAL-H, -78 °C 2. H₂O* 1. LiAlH4 2. H₂O+ 1. LiAlH4 J. OH 2. H₂O*arrow_forward12. Which reagents/conditions would lead to the transformation depicted below in high yield? a. NaOMe, MeOH b. MeOH, H₂O* c. NaOEt, EtOH 13. In the structure of 2-fluoropropanoic acid shown, the oxidation number on C1 is the oxidation number on C2 is a. +1, +2, -3 b. -3, +1, +3 d. NaOH, H₂O e. two of these , and the oxidation number on C3 is, c. 0, 0, 0 d. +3,0,-3 e. +2,0, -2 ??? OH 14. What will be the outcome of the following reaction sequence? B. 1. NaOEt, EtOH 2. PCC 3. isopropylMgBr 4. H₂0¹ ??? a. 2-ethyl-3-isopropyl-2,3-dimethyloxirane b. 2,3,4-trimethyl-3,4-epoxyhexane c. 1,2,3,4-tetramethyl-2,3-epoxypentane d. Both a and b are suitable. e. All of the above are correct. OH 15. What is a suitable IUPAC name for the structure shown at the right? OH D. F H OH LOH CH3 3 OHarrow_forward
- 6. Predict the product for the following reaction. "NH₂ Br H₂N NO₂ D. H₂O* A. Fe/HCI IV OH OH Br 11 NH₂ "NH₂ H₂N A. IB. II C. III D. IV E. V 7. Provide the reagents necessary to carry out the following reaction. NH₂ V 1. LIAIH4 2. H₂O NH₂ B. H₂CCOCI C. Br₂/FeBr3 E. dil. Na₂CO3 IIIarrow_forwardgive major productarrow_forwardFrom the table of reagents shown below, show how you synthesize the product from the given reactant. H3C NO₂ a. NBS, (PhCO₂), b. Bra, FeBr, c. Br₂, H d. OH e. NaOH(s), A 1. HNO₂, H₂SO 9. H₂ CrO Answer: NO₂ Reagents available. h. CH, CI, AICI, o. CuCN 1. CH₂ CH₂ Br p. H₂O, A 1. CH₂O, A q. CH₂ CH₂ COCI, AICI, k. CH, COCI, AICI r. H₂NNH,, OH A 1. Mg, Et₂0 s. H₂. Pd/C m. CO₂ (s) then H₂0 t. H₂PO, n. HONO 0°C u. Cl₂, FeCla (Enter the letter(s) of the reagent(s) needed in the box, in the order that they must be used. No more than two steps are required to perform the synthesis. Note that a retrosynthetic arrow is used, and therefore the reactant is shown on the right.) Previous Nextarrow_forward
- would you pls answer these 2? tnx so k mucharrow_forwardReagents CH3 a. HNO3, H2SO4 f. CH3OH LOCH3 b. SnCl2, H3O+ then NaOH(aq) g. CuCN C. HNO2, HCI h. Nal d. HBF4 i. LIAIH4 then H₂O e. KMnO4 j. CH3COCI Choose reagents from the table to synthesize this compound from toluene. Enter the letters in the order that you wish to use the reagents, without spaces or punctuation, i.e. 'adeg'. Submit Answer Try Another Version 2 item attempts remaining Visitedarrow_forwardPREDICT THE PRODUCT 1. BH3 2. H₂O2, NaOH 3. PCC 4. PhMgBr 5. H3O+ workup 1. H₂O, H₂SO4 2. PCC 3. CH3CH₂MgBr 4. H3O+ workup 1. mCPBA 2. CH3MgBr 3. H3O+ workup 4. NaH 5. CH31arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY