
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Show work. don't give Ai generated solution
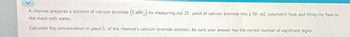
Transcribed Image Text:A chemist prepares a solution of calcium bromide (CaBr2) by. measuring out 25. μmol of calcium bromide into a 50. mL volumetric flask and filling the flask to
the mark with water.
Calculate the concentration in μmol/L of the chemist's calcium bromide solution. Be sure your answer has the correct number of significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What is the difference between (a) mass and density? (b) an extensive and an intensive property? (c) a solvent and a solution?arrow_forwardBeakers (a), (b), and (c) are representations of tiny sections (not to scale) of mixtures made from pure benzene and pure water. Select which beaker gives proper representation of the result when the two pure substances are mixed.arrow_forward1.87 A solution of ethanol in water has a volume of 54.2 mL and a mass of 49.6 g. what information would you need to look up and how would you determine the percentage of ethanol in this solution?arrow_forward
- 1.89 Imagine that you place a cork measuring 1.30cm4.50cm3.00cm in a pan of water. On top of this cork, you place a small cube of lead measuring 1.15 cm on a side. Describe how you would determine if the combination of the cork and lead cube will still float in the water. Note any information you would need to look up to answer the question.arrow_forwardSuppose that you are closing a cabin in the north woods for the winter and you do not want the water in the toilet tank to freeze. You know that the temperature might get as low as 30. C, and you want to protect about 4.0 L water in the toilet tank from freezing. Calculate the volume of ethylene glycol (density = 1.113 g/mL; molar mass = 62.1 g/mol) you should add to the 4.0 L water.arrow_forwardSpecific gravity is a physical property. Beakers hold three clear, colorless liquids A, B, and C. The values of the specific gravities of the liquids are measured and then listed in the Before freezing column. The beakers are placed in a freezer until a solid crust forms across the surface of each. The beakers are placed in a freezer until a solid crust forms across the surface of each. The crusts are removed, and the liquids are warmed to room temperature. Their specific gravities are measured again, and then their values are listed in the After Freezing column. Which beakers contains a pure substance, and which contain a mixture? Explain your reasoningarrow_forward
- The photograph below shows a beaker of water and a sugar cube, the combined mass of which is balanced by the weights on the right pan. The sugar cube is then placed in the water, and it dissolves completely. Do weights need to be added to or taken away from the right pan to keep the system in balance? Explain.arrow_forwardTo determine the average mass of a popcorn kernel, you collect the following data: Plot the data with number of kernels on the x-axis and mass on the y-axis. Draw the best straight line using the points on the graph (or do a least- squares or linear regression analysis using a computer program), and then write the equation for the resulting straight line. What is the slope of the line? What does the slope of the line signify about the mass of a popcorn kernel? What is the mass of 20 popcorn kernels? How many kernels are there in a handful of popcorn with a mass of 20.88 g?arrow_forwardWhat phases or states of matter are present in a glass of bubbling carbonated beverage that contains ice cubes?arrow_forward
- Please put the right amount of significant figuresarrow_forwardA chemist prepares a solution of barium chloride BaCl2 by measuring out 44.0μmol of barium chloride into a 150.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's barium chloride solution. Round your answer to 3 significant digits.arrow_forwardA chemist prepares a solution of barium chlorate (Ba(C10;),) by measuring out 119. umol of barium chlorate into a 350. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmol/L of the chemist's barium chlorate solution. Round your answer to 3 significant digits. mmol ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning



Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning