
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
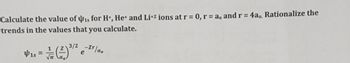
Transcribed Image Text:Calculate the value of is for H+, He+ and Li+2 ions at r = 0, r = a, and r = 4a,, Rationalize the
trends in the values that you calculate.
3/2 -Zr/ao
41s =
e
Υπ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 3arrow_forwardFrom the data in Table 14.2, predict B for DCl D is 2H.arrow_forwardThe ionisation energy of potassium is 4.34 eV and the electron affinity of chlorine is 3.61 eV. The Madelung constant for the KCl structure is 1.748 and the closest distance between ions of opposite sign is 0.314 nm. On the basis of these data, calculate the cohesive energy of KCl. Compare this with the observed cohesive energy of 6.42 eV for the ion pair and comment on the reasons for any discrepancyarrow_forward
- H2 has equilibrium bond length of 0.751 Å and bond dissociation energy of 432 kJ mol21, whereas F2 has equilibrium bond length of 1.417 Å and bond dissociation energyof 155 kJ mol21. On the same graph show qualitativesketches of the effective potential energy curve Veff for anH2 and an F2 molecule. In your solution, show the conversion from kJ mol21to the energy of a single molecule.1arrow_forwardThree biological ly important diatomic species. eitherbecause they promote or inhibit life. are (a) CO. (b) NO. and(c) CN-. The first binds to haemoglobin. the second is a neurotransmitter. and the third interrupts the respiratory electrontransfer chain. Their biochemical action is a reflection of theirorbital structure. Deduce their ground-state electron configurations. For heteronuclear diatomic molecules. a good firstapproximation is that the energy level diagram is much thesame as for homonuclear diatomic moleculesarrow_forwardIn a gaseous KCl molecule, the equilibrium internucleardistance is 2.67 × 10=10 m. Using data from Appendix Fand assuming that a Coulomb potential is a good approximation of the potential from K+ + Cl= to 2.67 3 10=10 m,estimate the dissociation energy of gaseous KCl into K andCl atoms (in kJ mo=1). (Note: Table 3.7 shows that aCoulomb potential is only a good approximation to thepotential between two charged atoms at long inter-atomicdistances; the percent ionic character of KCl at equilibrium is only 82%.)arrow_forward
- Based on data in Table 8.1, estimate (within 30 kJ>mol) thelattice energy for (a) LiBr, (b) CsBr, (c) CaCl2.arrow_forward18 19 20 21 22 23 A student proposes the following Lewis structure for the nitronium (NO₂) i ion. [0=N=0:1 Assign a formal charge to each atom in the student's Lewis structure. atom formal charge left O 1 N 0 right O 7 Kindergarten O....docx Continue Two Column N....pages X ✓ C. Grey Black and B....pdf 24 25 ©2022 M Grey Black and B....parrow_forwardA normal mode of vibration for H₂O is at 3650 cm Calculate the force constant, k, for H₂O. Assuming the force constant to be the same for H₂O and D₂O. Do you expect the corresponding D₂O wave number to be higher or lower? (Given: The atomic masses for H, D and O are 1.0, 2.0 and 16.0 respectively) (ii)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning