
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
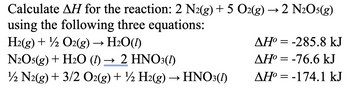
Transcribed Image Text:Calculate AH for the reaction: 2 N2(g) + 5 O2(g) →2 N2O5(g)
using the following three equations:
H2(g) + O2(g) → H₂O(1)
N2O5(g) + H2O (1) → 2 HNO3(1)
12 N2(g) + 3/2 O2(g) + ½ H2(g) → HNO3(1)
AH°=-285.8 kJ
ΔΗ° = -76.6 kJ
AH°=-174.1 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 2. What is the theoretical yield of Compound Y if you have 8.55 g of Reactant X reacted with an excess of Cr2O72? Balance the chemical equation. OH Compound X (a) OH + Cr₂O72- OH ° Compound Y OH + Cr³+ Fill in the squares in the following chemical equations: NaOH/H₂O EtOHarrow_forwardComplete and balance each of the following equations. If no reaction occurs, write no reaction. 1. NaCl(aq)+Hg2(C2H3O2)2(aq)→ 2. NaC2H3O2(aq)+Pb(NO3)2(aq)→arrow_forwardHNO3(aq) + Fe(OH)3(s) →arrow_forward
- NO, 3 NaOH 100°C NO2arrow_forwardWrite the Keq for: 2 K3PO4(aq) + 3 Ca(NO3)2(aq) ⇒ 6 KNO3(aq)+ Ca3(PO4)2(s)arrow_forwardCalculate the Enthalpy Change (ΔH) from average bond energies, which have been listed below in KJ/mol, for the following reaction and identify the nature of the reaction: CH3COOH + CH3OH → CH3COOCH3 + H2O [C‒H: 413; C‒C: 347; C=O: 745; C=C: 614; Cl‒Cl: 239, C‒O: 358; O‒H: 467]arrow_forward
- Acetylene is the substance burned in oxy-acetylene torches.Write a balanced equation for the complete oxidation reaction that occurs when acetylene (C2H2) burns in air.Use the smallest possible integer coefficients.arrow_forwardPredict the products of the following reaction. If no reaction will occur, use the NO REACTION button. Be sure your chemical equation is balanced! CaCO,(s) + HBr(aq) I S O+0 NO REACTIONarrow_forwardComplete and balance each of the following equations. If no reaction occurs, enter NOREACTION. a) Ca(NO3)2(aq)+KCl(aq)→ b) NaCl(aq)+Mg(C2H3O2)2(aq)→ c) NaCl(aq)+Mg(C2H3O2)2(aq)→ d) Al2(SO4)3(aq)+AgNO3(aq)→arrow_forward
- Predict the products of the following reaction. If no reaction will occur, use the NO REACTION button. Be sure your chemical equation is balanced! Rb(s) + H,O(1) → I NO REACTIONarrow_forwardPlease don't provide handwritten solutionarrow_forwardWrite the law for this reaction and explain how it is determined SO2CL2 ->SO2 + CL2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
