
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:© Macmillan Learning
Resources
Hint
Submit Answer
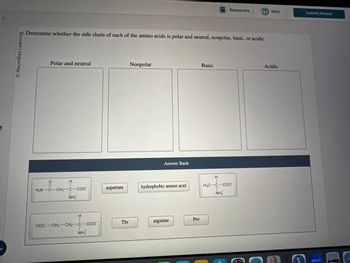
Determine whether the side chain of each of the amino acids is polar and neutral, nonpolar, basic, or acidic.
Polar and neutral
Nonpolar
Answer Bank
Basic
H
H
H2N C-CH2-C-COO
aspartate
hydrophobic amino acid
H3C-C-COO
NH3
NH3
H
OOC-CH2-CH2-C-COO
Thr
arginine
Pro
NH
Acidic
zoom
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- What special role does the amino acid cysteine have in the peptides vasopressin and oxytocin?arrow_forwardFor the tripeptide GlyAlaCys a. What amino acid is located at the peptides N-terminal end? b. What amino acid is located at the peptides C-terminal end? c. How many peptide bonds are present? d. How many amide linkages are present?arrow_forward22-44 How can a protein act as a buffer?arrow_forward
- For the tripeptide SerArgIle which amino acid residues a. are hydrophilic b. are hydrophobic c. possess polar neutral R groups d. participate in two amide linkagesarrow_forward22-89 What kind of noncovalent interaction occurs between the following amino acids? (a) Valine and isoleucine (b) Glutamic acid and lysine (c) Tyrosine and threonine (d) Alanine and alaninearrow_forward22-53 Do iron and zinc ions play role in protein structure? If so, what is the role for either or both?arrow_forward
- For the tripeptide AlaValGly which amino acid residues, if any, a. are hydrophilic b. are hydrophobic c. possess nonpolar R groups d. participate in two amide linkagesarrow_forwardChoose from A-F. Two of this amino acid can react with each other (anoxidation reaction) and form a covalent bond. CO- COO COO H3N-C-H H,N-C-H CH2 H2N CH 2 Н-С—он CH2 H2C CH2 CH3 CH3 Choice "A" Choice "B" Choice "C" Coo H,N-C-H H,N-C-H H3N-C-H CH2 CH2 C=CH NH CH2 C-NH | CH SH C-N H Choice "E" Choice "F" Choice "D"arrow_forward2. Classify the following amino acids as nonpolar, polar basic, polar acidic, or polar neutral. (a) (b) (c) (d) H₂N-CH-COH CH-OH I CH3 || H₂N-CH-C-OH I CH₂ C=O 1 OH H₂N-CH-C-OH CH₂ T CH-CH3 CH₂ H₂N-CH-C-OH ī CH₂ OH 229arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning