
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
How do I solve this?
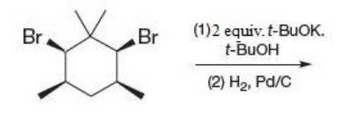
Transcribed Image Text:Br
Br
(1)2 equiv. t-BuOK.
t-BuOH
(2) H2, Pd/C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Cou X A Ch 6 x C Sear X * Sear X Sear x b > Hov x2 Con x Con X b Sear X y! cher X M Inbc X M Inbc X + Vent X 8 https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id3D15033867 + Sapling Learning macmillan learning Ch 6 Homework Jason Bauer , Sapling Learning > Ventura College - Chem V30 (31510) - Spring21 - ALAWDI > Activities and Due Dates > Ch 6 Homework E 20 of 24 Questions O Assignment Score: O Resources O Hint 68.3% Check Answer O 16 Question 100% x2 Question 20 of 24 > 1 of 5 Attempts Correct How many moles of CaCl2 are in 7.76 x 1024 formula units? O 17 Question 100% x2 1 of 5 Attempts Correct 7.76 x 1024 formula units = mol O 18 Question 100% x2 2 of 5 Attempts Correct O 19 Question 1 of 5 Attempts 100% x2 Correct 20 Question 0% O of 5 Attempts 21 Question 0% x2 O of 5 Attempts © 2011-2021 Sapling Learning, Inc. about us careers privacy policy terms of use contact us help 1:42 PM P Type here to search 99+ 2/18/2021 19arrow_forwardCH3 О А OB ос D H3C H3C A C Brz CH₂Cl₂ .Br H/I!! Н ? H3C H3C, B 1114 D H "/Br H "//Brarrow_forwardIdentifying organic functional groups compound - CH₁₂ - · CH3 N CH3 CH2 O C C- - CH3 family amine - - H ☐ - - CH3 — CH₂ — CH, || C. OH П CH3 - C - NH₂ ☐ Press (fn) F to exit full screen 000 Ararrow_forward
- ::&;;$(&;&;&;;arrow_forward5, Sect. 1-2) ot ot ot 1 pt pt pt pt 2 II. W X 18 12.14 Submit Answer tv E An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer. D C Ơ S C S Try Another Version R Z A 15.3 g piece of nickel was heated to 99.7 °C in boiling water and then dropped into a beaker containing 44.4 g of water at 24.6 °C. When the water and metal J/g. K came to thermal equilibrium, the temperature was 27.2 °C. What is the specific heat capacity of nickel? The specific heat capacity of water is 4.184 J/g. K. V S Document3.docx % 5 FOCUPSIA Pinerene ILOMMT wenarchive T 9 item attempts remaining east.cengagenow.com G B C Online t... H MacBook Pro 87 [References] N Oct 10. 2022 at 6:04 AM ● C AUD 75 71177 21 11.54 AM J 00* 8 M C K G x WOO 10 KB Micros...(.docx) mand Email Instructor Previous O C Next 30 Save and Exit O Tue O O الد C Viaarrow_forward||| 1. Brz (1ev). 2. D₂, Pd/c me OHarrow_forward
- Which of the following structures is consistent with the IR spectra shown below? % Transmittance 80 60 40 20 3500 3000 2500 wmying 2000 1500 1000 500arrow_forwardRCO3H 1) Mg Br 2) CO₂ b) 3) H c) CO₂H 1) LiAlH4 2) H3O+ d) SOCI2 CO₂H (CH3)2CuLi CI 1) LIAI(OR)H, f) CI 2) H* g) h) J) H CO2H + CH3OH O-CH3 1) CH3MgBr (2 equiv.), 2) H3O+ CN 1) CHзMgBr 2) H₂O* CN 1) LiAlH4 2) H₂Oarrow_forwardFill in the missing information. ОН CH3 H+ НО Br- ОН m-CPBA ОН Br H2 Pd/C 1. NaBH4 2. H2O+ Іarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning