
Concept explainers
Benzaldehyde is produced from toluene by the following catalytic reaction (see figure):
Dry air and toluene vapor are fed to the reactor at 350 F and 1 atm. Air is supplied 100% in excess. Of the toluene that is fed to the reactor, 13% reacts to form benzaldehyde and 0.5% reacts with oxygen to form CO2 and H2O. The gases produced leave the reactor at 379 F and 1 atm. Water is circulated through the jacket surrounding the reactor, and it enters at 20 ° C and exits at 55 ° C.
Calculate:
a. Moles of benzaldehyde formed (mol)?Note: Do not use decimals for your answer.
b. Moles of toluene that did not react (mol)?
c. Moles of water generated (mol)?
d. Moles of carbon dioxide generated (mole)?
e. Moles of oxygen that do not react (mol)?
f. Reactor heat transfer rate (kJ / h)
g. Cooling water flow rate (cm3 / h)
Data:
Δhvap benzaldehyde (179 ° C) = 38.40 kJ / mol
Cp benzaldehyde (Liq) = 59.42x10-2 kJ / mol
Cp benzaldehyde (vap) = 40.05x10-2 kJ / mol
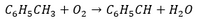
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

- Consider the reaction of aluminum metal with hydrochloric acid. 2 Al(s) + 6 HCl(aq) → 2 AlCl3(aq) + 3 H₂(g) When 5.00 g of aluminum is added to an excess of hydrochloric acid, all at a constant pressure of 1 atm and a constant temperature of 298 K, the system expels 99.8 kJ of heat into the surroundings. O Calculate the number of moles of hydrogen gas that are produced. Assume that the heat is exchanged without a change in temperature. calculate the amount of work done by the system when the hydrogen gas expands against a constant-pressure atmosphere of 1 atm at 298 K. (Use R = 8.314 x 10³ kJ mol-¹ K-¹.) Use the sign conventions of heat and work and the First Law of Thermodynamics calculate the change in internal energy of the system.arrow_forwardIf a polymer sample contains an equal number of moles of species with degrees of polymerization x = 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10, what are the number-average and weight-average degrees of polymerization ? [Ans. DP = 5.5; DP, = 7] EXERCISES 4.1 4.2 One gram of polymer A (DPn = 1000, DP = 2000) is mixed with 2 g of polymer B (DP = 2000, DP,= 5000). Calculate the degree of polymerization of the mixture that would be derived from osmotic pressure measurements at several concentrations. [Ans. DPn = 1500; DPw = 4000] %3D 4.3 Polyethylenes A, B, and C from three sources are to be blended to achieve a weight- average molecular weight of 210,000 and polydispersity index (PDI) of 3.0. How much of each polymer should be used to obtain 10,000 kg blend ? Weight-average mol. wt. 130,000 Polyethylene PDI A 2.50 2.00 220,000 400,000 [Hint: Mw = Ewi(Mw)i; 1/Mn = Ewi/(Mn)i; PDI = Mw[Mn.] [Ans. A = 1892 kg, B = 5776 kg, C = 2332 kg; (Total 10,000 kg)] 2.50 %3D 4.4 Polymer samples A and B are…arrow_forwardAmmonia is oxidized in a continuous reactor 4NH3 g + 5O2 g --> 4NO g + 6H2O g delta AHr= -904.7KJ mol The feed stream (40 mole% NH3 and 60 mole% O2 )enters the reactor at 200 degrees celsius with 80% conversion of ammonia and the products leave at 300 degrees celsius. Determine the quantity of heat required to be added or removed from the reactor.arrow_forward
- Use the chemical reaction below to answer the following questions HCI(aq) + NaOH(aq) → NaCl (aq) + H2O (I) AH298 = -58 kJ/mol (a) How much heat is produced when 200 mL of 0.3 M HCL (density = 1.00 g/mL) and 250 mL of 0.25 M NaOH (density = 1.00 g/mL) are mixed? (b) If both of the solutions at the same temperature and the heat capacity of the products are 5.39 J/g °C , how much will the temperature increase? (c) What assumptions are made when calculating your answersarrow_forwardFollow the standard energy balance steps to determine the water condensed and the heat removed from a humid air stream. The feed stream contains 12 wt% water (balance is air), has a temperature of 250°C and a pressure of 1 atm. The exit gas stream is at 1 atm and 25°C. The exit vapor composition should be determined from the psychrometric chart (saturated). The heat of vaporization of water at 25°C is 44.0 kJ/mol. Draw a picture and label appropriately (Basis: 1 kg/s feed). a. b. Perform a degree of freedom analysis. c. Write appropriate equations. d. Complete all material balances. e. Specify reference states.arrow_forwardDO NOT SOLVE PROBLEM. Please just calculate extent of reaction and flow rates of each component in the reaction.arrow_forward
- Follow the standard energy balance steps to determine the water condensed and the heat removed from a humid air stream. The feed stream contains 18 wt% water (balance is air), has a temperature of 200°C and a pressure of 1 atm. The exit gas stream is at 1 atm and 30°C. The exit vapor composition should be determined from the psychrometric chart (saturated). The heat of vaporization of water at 30°C is 43.8 kJ/mol. a. Draw a picture and label appropriately (Basis: 1 kg/s feed). b. Perform a degree of freedom analysis. c. Write appropriate equations. d. Complete all material balances. e. Specify reference states. f. Construct and fill out enthalpy table. Define variables for unknown enthalpies. Species/Phase min (kg/s) H₂O (v) Air (g) H₂O (1) g. Determine unknown enthalpies. ĤIn (kJ/mol) mout (kg/s) Hout (kJ/mol)arrow_forwardA countercurrent extraction system uses 3 steps for acid extraction acetic acid/water/isopropyl ether at 20°C and 1 atm. The feed consists of 40% (by mass) of acetic acid and 60% (by mass) of water. The feed is 2000 kg/h. The solvent used contains 1% (by mass) of acetic acid. It is necessary that the raffinate at the exit of the extractor contains 5% of acetic acid (by mass). Determine: The amount of solvent, extract in MS and raffinate R1 Equilibrium data: Equilibrium diagrams of the ternary system: acetic acid/water/isopropyl ether(in mass fractions at 20°C and 1 atm).arrow_forwardA fuel gas of 73 mol% ethane and the balance propane is burned continuously with 69% excess air to provide energy to a boiler to generate steam. In the combustion process, 86% of the ethane and 75% of the propane are burned. If 22 mol/s of the fuel gas are fed to the combustion chamber at 1 atm and 25 C, the product gases emerge at these same conditions, what is the maximum rate of heat supply to the boiler (positive quantity) in MW (1 MW = 1 megawatt = 106 W)? Assume the H2O produced in the combustion process leaves as liquid water and the rest of the components are in the gas/vapor phase. Show all work, cite all sources, state all assumptions. Report answer in MW with 3 digits of precision in decimal format.arrow_forward
- Ammonia is oxidized in a continuous reactor 4NH3 g + 5O2 g --> 4NO g + 6H2O g delta AHr= -904.7KJ mol The feed stream (40 mole% NH3 and 60 mole% O2 )enters the reactor at 200 degrees celsius with 80% conversion of ammonia and the products leave at 300 degrees celsius. Determine the quantity of heat required to be added or removed from the reactor. The basis of calculation: 10mol/s of feed stream. given: the constant heat capacity, Cp, of NH3(g), O2(g), NO(g), and H2O(v) are 0.035, 0.029, 0.029 and 0.033KJ/ mol. degrees celsius respectively.) Cp O2 or table 8 for the calculation. can you please explaing each steparrow_forwardprovide step by step solution. Show every step. Assume the reader is learning from youarrow_forward021 A cylinder contains a mixture of Ethane ( CH, ) and air and the of air to fuel is (A/F= 19.2/1) at 300 ° C. The internal ratio energy of Ethane is equal (AU. = - 47590 kJ/mole) at (25 °C ). when the full combustion is at constant pressure and the temperature of the products is (3250°C). Calculate the heat transferred from the combustion. Take specific heat : Reactants ( C2H6 = 1.7, 02= 22.3, N2= 21.2 KJ / mole.K ) Products (CO2= 46.5, H20(Vap)= 27.3, 02=25.5 KJ / mole.K), R = 8.314 KJ / mole.K.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





