
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
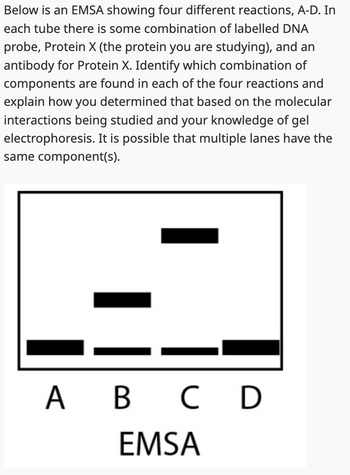
Transcribed Image Text:Below is an EMSA showing four different reactions, A-D. In
each tube there is some combination of labelled DNA
probe, Protein X (the protein you are studying), and an
antibody for Protein X. Identify which combination of
components are found in each of the four reactions and
explain how you determined that based on the molecular
interactions being studied and your knowledge of gel
electrophoresis. It is possible that multiple lanes have the
same component(s).
A B C D
EMSA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A purified recombinant protein is analyzed for molecular weight by SDS-PAGE at pH 8.5. From the protein sequence deduced from the gene that was expressed in bacteria, the protein is expected to have a molecular weight of 44,000. However, the molecular weight of the protein is found by SDS-PAGE to be 52,000. Explain the reason or reasons for this difference in molecular weight. What calculation could you make to help explain this discrepancy?arrow_forwardStreptococcus pneumoniae cells of genotype str s mtl - are transformed by donor DNA of genotype strr mtl+ and (in a separate experiment) by a mixture of two DNAs with genotypes strr mtl - and str s mtl+. The accompanying table shows the results.a. What does the first row of the table tell you? Why? b. What does the second row of the table tell you? Why?arrow_forwardSDS-PAGE and Agarose gel electrophoresis can both be used to separate proteins or protein- complexes based on their size. Separation of a multi-subunit protein complex by SDS-PAGE resulted in two bands with molecular weights of 86 KDa and 136 KDa, while separation of the same complex using Agarose gel electrophoresis resulted in two bands with molecular weights of 222 KDa and 444 KDa. Based on this information, which of the following statements is most likely to be correct? O The complex has a molecular weight of 222 KDa O The complex exists as a dimer of homo-dimers O The complex exists as a dimer of hetero-dimers SE O The complex exists as a trimer, but the individual protein subunits are covalently crosslinked to each otherarrow_forward
- Monoclonal antibodies can be conjugated to an insoluble support by chemical methods. Explain how these antibody-bound beads can be exploited for protein purification.arrow_forwardA bloactlve hexapeptide was cleaved by trypsln from a larger proteln. The peptlde was sequenced by treatment with varlous reagents and enzymes. The results are as follows: a) Treatment wlth cyanOgen bromlde gave a tetrapeptlde of compositlon asp, trp, cys and met. b) Treatment with chymotrypsln gave a tripeptlde of compositlon asp, trp and cys, a dipeptlde wlth the same C-term and an amlno acld. c) Treatment with streptococcal protease gave a dipeptlde and a tetrapeptlde. The composltion of the peptlde Is lys, trp, asp, cys and met 1. What Is the sequence of the hexapeptlde? 2. What is the pl of thls peptlde?arrow_forwardGel Electrophoresis A. Why does DNA travel toward the positive electrode in the gel chamber? B. What is a ladder sequence and why is it useful to include in a gel?arrow_forward
- Various antimicrobial drugs to treat microbial infection have diverse mechanism of action. Consider the following antimicrobial drugs: A. Seconeolitsine, known as DNA topoisomerase I inhibitor in bacteria. (i) Explain briefly how inhibiting DNA topoisomerase I is a good mechanism of action for an antibiotic, include possible molecular machineries being targeted. (ii) What would be an appropriate response if seconeolitsine works well by stating the state of supercoiling in bacteria. (iii) To prove your answer (ii), you test the condition of bacterial DNA by running gel electrophoresis, one has been treated with seconeolitsine (+ sample) and the other one is not (- sample). Explain the position of each + sample and – sample band on the gel in reference to the point of origin (where you load your samples) or how far each DNA sample travel across agarose gel. (iv) Explain why you would expect answer (iii) for each + sample and – sample. B.…arrow_forwardAnswer the following questions below:arrow_forwarda) What are the important parameters for the eluent selection in Gel Permeation Chromotography (GPC)? Explain your answer in detail. What is an universal calibration curve and what is the advantage of using it in GPC analysis?arrow_forward
- A plaque assay is performed beginning with 1 mL of a solution containing bacteriophages. This solution is serially diluted 3 times by combining 0.1 mL of each sequential dilution with 9.9 mL of liquid medium. Then 0.1 mL of the final dilution is plated in the plaque assay and yields 12 plaques.What is the initial density of bacteriophages in the original 1 mL? Enter your answer to two significant figures ( for example: 1.1 * 10^2)arrow_forwardA protein gives a single band on SDS gel electrophoresis, as shown in lanes 1 and 2 below. There is little, if any, effect from adding β-mercaptoethanol(BME) to the sample; if anything, the protein runs a little bit slower. When treated with the proteolytic enzyme thrombin and electrophoresis in the absence of BME, the protein migrates a bit more rapidly (lane 3). But if BME is present, two much more rapidly migrating bands are found (lane 4). Explain these results in terms of a model for the protein.arrow_forward1) The Nde1 enzyme comes at a concentration of 40 U/ul. According to the manufacturer, the container has enough for 10,000 reactions of 2 ug of DNA, assuming that 1 U/ug of DNA is recommended. How many units in total does the packaging contain? At what concentration in U/ml does the enzyme come? If I had to use 10 U for a reaction of 10 ugs of DNA, how would I serve it? 2) A serially diluted phage solution is used to inoculate an E coli plate in the double-layer plate assay. In 24 hours you count 148 plaques. Considering that the original stock was 0.1, and that you used the sixth dilution of the series, what is the PFU for that phage in this strain of E coli? What is the dilution factor for this plate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education