
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
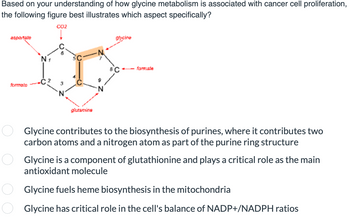
Transcribed Image Text:Based on your understanding of how glycine metabolism is associated with cancer cell proliferation,
the following figure best illustrates which aspect specifically?
CO2
aspartate
formate
оо
-C 3
glutamins
9
glycine
8C
--formate
Glycine contributes to the biosynthesis of purines, where it contributes two
carbon atoms and a nitrogen atom as part of the purine ring structure
Glycine is a component of glutathionine and plays a critical role as the main
antioxidant molecule
Glycine fuels heme biosynthesis in the mitochondria
Glycine has critical role in the cell's balance of NADP+/NADPH ratios
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Why does activation of the phosphorylated b form of glycogen synthase by high concentrations of glucose 6-phosphate make good biochemical sense? It ensures that glucose is saved when it is abundant and not being used to fuel energy metabolism. It ensures that excess fuel is stored in the most reduced and energy-rich form. It prevents glycogen from being synthesized at the same time as it is being broken down. It prevents the mobilization of glycogen when glucose is already abundant.arrow_forwardThe phosphorylation and oxidative decarboxylation of oxaloacetate by inorganic phosphate (Pi) to make phosphoenolpyruvate and carbon dioxide is endergonic under intracellular conditions. It is characterized by this equation: Oxaloacetate + Pi ←→ Phosphoenolpyruvate + H2O + CO2 ΔG’ = +24.6 kJ/mol The synthesis of GTP from GDP and inorganic phosphate (Pi) in solution is endergonic under intracellular conditions, and it is characterized by this equation: GDP + Pi ←→ GTP + H2O ΔG’ = +30.5 kJ/mol Write a new net thermodynamically coupled reaction equation that describes the synthesis of phosphoenolpyruvate from oxaloacetate using the hydrolysis of GTP to power the reaction and calculate the new net ΔG’ of this reaction. Show all of your work.arrow_forwardFor each of the following proteins describe how the protein function would be altered (or not) in the following scenarios: A receptor tyrosine kinase that always dimerizes even in the absence of ligand 3-ketoacyl-CoA transferase (SCOT) that is expressed in the liver. CPT1 that doesn’t have a malonyl binding site 4. acylCoA dehydrogenase with complex V inhibitedarrow_forward
- Insulin resistance, as occurs in type 2 diabetes, may lead to increased ketone production and release into blood. Describe the biochemistry that links insulin resistance and ketone production. Compare the cellular energy (e.g. ATP) required and produced when glycogen is synthesize and hydrolyzed, respectively. Compare and contrast the mechanism of fatty acid synthase with translation. When young rats are placed on a totally fat acid free diet, they grow poorly, develop a scaly dermatitis, lose hair, and soon die these symptoms that can be prevented if plant material is included in the diet. Why?arrow_forwardDescribe how a) Mean Arterial Pressure, b) Maximal Oxygen Consumption, and c) Blood Flow are determined using its formula. In addition, you should explain what those factors are including cardiac output, stroke volume, heart rate, resistance, a-vO2 difference, etc. Describe how many ATPs can be generated by complete cycles of beta oxidation of free fatty acid with 20 carbons. You should indicate how many cycles of beta oxidation and Krebs cycle, and total number of products as wellarrow_forwardin the synthesis of glycogen, the glycogen synthase uses UDP-glucose rather than glucose. Explain in detail way.arrow_forward
- blank 1 choices: a decrease, no change, an increase blank 2 choices: decrease in, increase in, constant blank 3 choices: incresse, descrease, remain unchanged blank 4 choices: proceed, proceed noarrow_forwardThe rate of the reaction of glycogen N with inorganic phosphate, Pi, to form glucose-1-phosphate and glycogen N-1 is:arrow_forwardThe biosynthesis of vitamin D₂ is a two-step reaction, requiring two types of concerted pericyclic reactions. Draw in the arrows for each step and identify the structure of precalciferol. If an electrocyclic reaction is required, identify if it occurs through a conrotatory or disrotatory reaction. HO H H ergosterol hv precalciferol Cast HO ergocalciferolarrow_forward
- Which of an alpha-D-2,3-di-O-methylglucopyranose or alpha-D-2,3, 6-tri-O-methylglucopyranose represents a glucose unit in glycogen which was originally carrying an (alpha 1-->6) glycosidic bond?arrow_forwardCompare and contrast the c4 pathway and the malate–aspartate shuttle in bullet format, meaning list the similarities and differences.arrow_forwardThe formation of thymidylate is typically coupled with the synthesis of which amino acid? O Cysteine Glutamate Alanine Serine O Glycine O Glutaminearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON