
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![< PREV
1
2
3
NEXT >
Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka
in order to determine the unknown. Each reaction participant must be represented by
one tile. Do not combine terms.
Ка
=
RESET
[0]
[0.95]
[0.68]
[8.68]
[x]
[2x]
[2x]²
[0.95+x]
[0.95 - x]
[0.95 + 2x]
[0.95 -2x]
[0.68+x]
[0.68 - x]
[0.68 + 2x]
[0.68 - 2x]
8.68
0.939
4.79 × 10-*
2.09 × 10-º
< PREV
1
2
3
Based on your ICE table (Part 1) and the equilibrium expression for Ka (Part 2),
determine the pH of the buffer solution.
pH
RESET
0
19.65
5.46
0.95
0.022
8.53
3.4 × 10-€
2.9 × 10-9](https://content.bartleby.com/qna-images/question/f922ab2d-c754-4c9c-9d3b-bd784fecfd89/103f0041-33fc-458d-b44e-781d5652f2f5/cvkmr8a_thumbnail.png)
Transcribed Image Text:< PREV
1
2
3
NEXT >
Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka
in order to determine the unknown. Each reaction participant must be represented by
one tile. Do not combine terms.
Ка
=
RESET
[0]
[0.95]
[0.68]
[8.68]
[x]
[2x]
[2x]²
[0.95+x]
[0.95 - x]
[0.95 + 2x]
[0.95 -2x]
[0.68+x]
[0.68 - x]
[0.68 + 2x]
[0.68 - 2x]
8.68
0.939
4.79 × 10-*
2.09 × 10-º
< PREV
1
2
3
Based on your ICE table (Part 1) and the equilibrium expression for Ka (Part 2),
determine the pH of the buffer solution.
pH
RESET
0
19.65
5.46
0.95
0.022
8.53
3.4 × 10-€
2.9 × 10-9
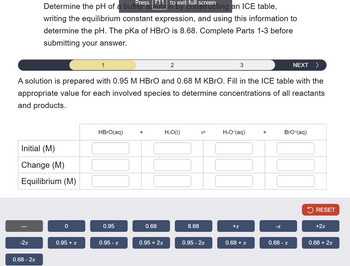
Transcribed Image Text:Press | full screen
Determine the pH of a buffs 11 to exit furting an ICE table,
writing the equilibrium constant expression, and using this information to
determine the pH. The pKa of HBrO is 8.68. Complete Parts 1-3 before
submitting your answer.
1
2
3
NEXT >
A solution is prepared with 0.95 M HBrO and 0.68 M KBRO. Fill in the ICE table with the
appropriate value for each involved species to determine concentrations of all reactants
and products.
HBrO(aq)
+
H₂O(1)
=
H3O+(aq)
+
BrO-(aq)
Initial (M)
Change (M)
Equilibrium (M)
0
0.95
0.68
8.68
+x
-x
RESET
+2x
-2x
0.95 + x
0.95 - x
0.95 + 2x
0.95 - 2x
0.68 + x
0.68 - x
0.68 + 2x
0.68 - 2x
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Chemistry Determine the pH of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. Complete Parts 1-3 before submitting your answer. NEXT > The buffer was prepared by dissolving 20.0 g NaCH,CO0 into a 500.0 mL solution of 0.150 M of CH,COOH. Assume the volume of the solution does not change. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. CH,COOH(aq) + H,O(1) H,O"(aq) + CH,COO (aq) Initial (M) Change (M) Equilibrium (M) 5 RESET 20.0 0.150 0.244 0.488 0.678 20.0+ 20.0- 0150 +1 0.150- 0.244+ 0.488 0.244- 0.488-x 0678 0.678-1 Determine the pH of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. Complete Parts 1-3 before submitting your answer. NEXT ( PREV The Ka for CH,COOH, is 1.8 x 10, Based on your ICE table (Part 1) and the…arrow_forwardThe dissociation constant of HNO, at 25 °C is Ka = 26.8. Find the percent dissociated in 0.100 M HNO, and in 1.00 M HNO, at 25 °C. 16.37 percent dissociated in 100 M HNO,: Incorrect 5.18 percent dissociated in 1.00 M HNO,: Incorrectarrow_forwardA Dashboard 101 Chem101 b Answered: At a particular temper X A aрp.101edu.co Question 3 of 23 Submit A 0.1000 M solution of a weak acid, HA, is 3.0% dissociated. Determine the value of Ka for the weak acid. 2 NEXT > Based on the given values, fill in the ICE table to determine concentrations of all reactants and products. НА(aq) H:O(1) H:O*(aq) A-(aq) + Initial (M) Change (M) Equilibrium (M) 2 RESET 0.1000 3.0 -3.0 0.030 -0.030 0.0030 -0.0030 0.0970 -0.0970 0.0097 -0.0097 + 10:34 PM P Type here to search 65°F Mostly sunny 10/31/2021arrow_forward
- Solve 4. investigate the qualitative and quantitative nature of chemicalsystems at equilibrium, and solve related problems.arrow_forward7. Refer to the following table: Table 2-2. Change in pH of 0.2 M Acetate Buffer after addition of Acid or Base [Base]:[Acid] 0.005:0.195 Addition pH HC1 0.095 mol 3.1 HC1 0.075 0.025:0.175 3.9 HС1 0.05 НС1 0.025 0.05:0.15 4.2 0.075:0.125 4.5 0.1:0.1 0.125:0.075 4.7 NaOH 0.025 mol 4.9 NaOH 0.05 mol 0.15:0.05 5.2 NaOH 0.75 mol 0.175:0.025 5.5 NaOH 0.095 mol 0.195:0.005 6.3 a. Calculate the pH of a 0.2 M acetate buffer (a solution containing 0.1 mol L-' acetic acid and 0.1 mol L-1 sodium acetate), given that the pKa of acetic acid is 4.7. b. What would be the pH value after adding 0.05 mol of NaOH to 1 L of 0.2 mol L1 acetate buffer? c. Compare the pH value in (b) with that obtained after adding 0.05 mol NAOH to 1 L of water (a simple solution of 0.05 mol L-' NaOH). d. How much acid or base can be added to 1 L of the 0.2 M acetate buffer so that the pH does not change appreciably (i.e., what is its buffer range)?arrow_forwardI need help answering number 5arrow_forward
- The final part asks for concentration of OH-arrow_forwardWhat is the answerarrow_forward12. Carbonated water has a [H+Jeg = 3.2 × 10-4 M; this is the result of the dissociation of carbonic acid: H₂CO3 (ag) H+ (aq) + HCO3- (ag) Kc = 4.4 x 10-7 a. Set up the equilibrium constant expression for this acid dissociation. b. Calculate [H₂CO₂]ea. Hint: An ICE table may be handy. c. Carbonic acid is created by dissolving carbon dioxide in water. Given that the Henry's Law constant of carbon dioxide is 29.76 atm/M, what partial pressure of carbon dioxide is needed to make carbonated water?arrow_forward
- 19. A student prepared a 250.0 mL solution by dissolving 4.559 g of (NH4)2SO4 in enough water. The temperature is 298 K. The base dissociation constant for NH3 is 1.796x10-5. Please answer the following questions. A. B. What is the equilibrium concentration of H3O+ in the solution? Please provider your answer below. 00 + → What is the equilibrium concentration of OH in the solution? Please provider your answer below. OO → $ → $ C. What is the pH of the solution? pH = Please provider your answer below. S M Marrow_forwardDetermine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HClO and 0.37 M NaClO. The value of Ka for HClO is 2.9 × 10⁻⁸ Please solve for the pHarrow_forwardAnswer using this data for the remaining questions on this page: For 1.0L of an acetic acid solution: Ka = 1.8 x 10-5 and [HA] = 0.250M 2. Calculate the pH of the solution. 3. What is the pka? 4. Calculate the pH of the solution if you make it a buffer by adding enough solid sodium acetate to make the concentration of acetate ion equal to 0.35M. The volume of the solution does not change. 5. What is the new concentration of [HA] if 2.0mL of 5.00M of HCI is added to 1.000L of the solution above? 6. What is the new pH?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY