
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
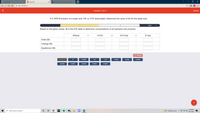
Transcribed Image Text:**ICE Table Problem for Weak Acid Dissociation**
**Question 3 of 23:**
*A 0.1000 M solution of a weak acid, HA, is 3.0% dissociated. Determine the value of Ka for the weak acid.*
**Instructions:**
Based on the given values, fill in the ICE (Initial, Change, Equilibrium) table to determine concentrations of all reactants and products.
**Chemical Equation:**
HA(aq) + H2O(l) ⇌ H3O+(aq) + A-(aq)
**ICE Table:**
| | HA(aq) | + | H2O(l) | ⇌ | H3O+(aq) | + | A-(aq) |
|---------------|--------|---------------|--------|----|----------|---------------|--------|
| Initial (M) | | | | | | | |
| Change (M) | | | | | | | |
| Equilibrium (M)| | | | | | | |
**Option Buttons:**
- -0.0030
- 0.0970
- 0.1000
- 3.0
- -3.0
- 0.030
- -0.030
- 0.0030
**Reset Button:** A reset option is available to start over if needed.
**Note:** Understanding the ICE table and the dissociation percentage is crucial in calculating the acid dissociation constant, \( K_a \), for the weak acid. The table should be used to track changes in molarity throughout the reaction to reach equilibrium.
![**Question 3 of 23**
A 0.1000 M solution of a weak acid, HA, is 3.0% dissociated. Determine the value of Ka for the weak acid.
**Instruction:**
Based on your ICE table and definition of Ka, set up the expression for Ka and then evaluate it. Do not combine or simplify terms.
\( K_a = \)
**Answer Options:**
- [0]
- [0.1000]
- [3.0]
- [0.030]
- [0.0030]
- [0.0970]
- [0.0097]
- [0.48]
- [2.5]
- [0.31]
- [9.3 x 10^-6]
- [1.1 x 10^-6]
- [3.2]
- [9.3 x 10^-5]
**Features:**
- “PREV” and “Submit” buttons available for navigating questions.
- A “RESET” button to clear selections.
- A progress bar indicating question 2 out of 23 is currently active.
Make sure to use the knowledge of equilibrium constants and ICE tables to select the correct values from the given options.](https://content.bartleby.com/qna-images/question/315f2cc5-843f-4088-b4c8-06378b03d38c/c21b214f-d8b6-44b8-b268-65d4a10a1519/bz4qltj_thumbnail.png)
Transcribed Image Text:**Question 3 of 23**
A 0.1000 M solution of a weak acid, HA, is 3.0% dissociated. Determine the value of Ka for the weak acid.
**Instruction:**
Based on your ICE table and definition of Ka, set up the expression for Ka and then evaluate it. Do not combine or simplify terms.
\( K_a = \)
**Answer Options:**
- [0]
- [0.1000]
- [3.0]
- [0.030]
- [0.0030]
- [0.0970]
- [0.0097]
- [0.48]
- [2.5]
- [0.31]
- [9.3 x 10^-6]
- [1.1 x 10^-6]
- [3.2]
- [9.3 x 10^-5]
**Features:**
- “PREV” and “Submit” buttons available for navigating questions.
- A “RESET” button to clear selections.
- A progress bar indicating question 2 out of 23 is currently active.
Make sure to use the knowledge of equilibrium constants and ICE tables to select the correct values from the given options.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine concentration of OH in a 0.724 M solution of BrO¯ (Kb = 4.0 x 10-6). 1 2 3 NEXT > Based on the given values, set up ICE table in order to determine the unknown. BrO (aq) H20(1) OH (aq) HBRO(aq) + + Initial (M) Change (M) Equilibrium (M) 5 RESET 0.724 4.0 x 10-6 0.362 +x +2x -x -2x 0.724 + x 0.724 - x 0.724 + 2x 0.724 - 2x 4.0 x 10-6 + x -9- 4.0 x 10-6 - x 4.0 x 10-6 + 2x 4.0 x 10-6 - 2xarrow_forwardif you have the dissolution of Mg(NO3)2 will adding the following cause the salt to dissolve or precipiate more: a) adding HCL b) adding Mg(NO3)2 c) adding solid Mg(NO3)2 d) adding KIO4arrow_forwardA1.arrow_forward
- For which of the following equilibria does K, correspond to the base-ionization constant, K, of HCO3 ? a) H2CO3(aq) + H2O(1) = HCO3 (aq) + H3O*(aq) O b) HCO3 (aq) + H2O(1) H2CO3(aq) + OH (aq) UC) HCO3 (aq) + H3O*(aq) = H2CO3(aq) + H20(1) O d) HCO3 (ag) + H2O(I) = CO3² (aq) + H3O*(aq) e) HCO3 (aq) + OH (aq) = CO3² (aq) + H20(1)arrow_forwardDetermine the pOH of a 0.200 M solution of a weak acid, HA, that is 3.0% dissociated at equilibrium. 1 2 NEXT > Based on the given values, fill in the ICE table to determine concentrations of all reactants and products. HA(aq) H:O(1) H:O"(aq) A"(aq) Initial (M) Change (M) Equilibrium (M) 5 RESET 0.200 3.0 -3.0 0.030 -0.030 0.0030 -0.0030 0.060 -0.060 0.0060 -0.0060 0.197 -0.197 0.194 -0.194 +x -Xarrow_forwardA 0.1000 M solution of a weak acid, HA, is 3.0% dissociated. Determine the value of Ka for the weak acid. 1 NEXT Based on the given values, fill in the ICE table to determine concentrations of all reactants and products. HA(aq) H,O(1) H,O(aq) A(aq) + Initial (M) Change (M) Equilibrium (M) 5 RESET 0.1000 3.0 -3.0 0.030 -0.030 0.0030 -0.0030 0.0970 -0.0970 0.0097 -0.0097arrow_forward
- ⒸMacmillan Learning Write the chemical reaction for chlorous acid in water, whose equilibrium constant is K₂. Include the physical states for each species. K₁ reaction: HCIO, (aq) + H₂O(aq) → H₂O¹(aq) + C1O₂ (aq) = Write the chemical reaction for the chlorite ion in water, whose equilibrium constant is K. Include the physical states for each species. Kreaction: CIO₂ (aq) + H₂O(aq) ⇒ HC10₂(aq) + OH¯(aq)|arrow_forwardSuppose a 250. mL flask is filled with 1.0 mol of Cl, and 1.8 mol of HCI. The following reaction becomes possible: H, (g) + Cl, (g) = 2HC1(g) The equilibrium constant K for this reaction is 5.89 at the temperature of the flask. Calculate the equilibrium molarity of H,. Round your answer to two decimal places. olo OM Ar Explanation Check © 2022 McGraw Hill LLC. AIl Rights Reserved. Terms of Use Privacy Center | Accessibilityarrow_forwardit's a chemistry hw needed asaparrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY