
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
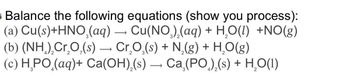
Transcribed Image Text:Balance the following equations (show you process):
(a) Cu(s)+HNO₂(aq) → Cu(NO)2(aq) + H2O(l) +NO(g)
(b) (NH) Cr₂O(s) → Сг₂O̟(s) + N2(g) + H₂O(g)
(c) HPO (aq)+ Ca(OH)2(s) Ca (PO)₂(s) + H₂O(1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forwardWrite a balanced equation for the reaction of hydroiodic acid, HI, with calcium hydroxide, Ca(OH)2. Then, write the balanced complete ionic equation and the net ionic equation for this neutralization reaction.arrow_forwardWhat is the molarity of a solution of sodium hydrogen sulfate that is prepared by dissolving 9.21 g NaHSO4 in enough water to form 2.00-L solution? What is the molarity of each ion in the solution?arrow_forward
- Given the reaction : 6KOH(aq) + Fe2(SO4 )3(aq)→ 2Fe(OH)3(s)+ 3K2SO4(aq) (14 pts) (a)Write the balanced complete ionic equation for the reaction (b)Write the balanced netionic equation for the reactionarrow_forwardWhat mass of sodium hydroxide, NaOH, would be required to produce 16 g of the antacid milk of magnesia [magnesium hydroxide, Mg(OH)2] by the following reaction? MgCl2(aq) + 2NaOH(aq) ⟶ Mg(OH)2(s) + 2NaCl(aq)arrow_forwardWhen placed in water, potassium starts to react instantly and continues to react with great vigor. On the basis of this information, select the better of the following two equations to represent the reaction. 2 K(s) + 2 H,O(e) –→ 2 KOH(aq) + H2(g) 2 K(s) + 2 H;O* (aq) → 2 K* (aq) + H,(g) + 2H,0(e) | State the reason for your choice.arrow_forward
- Given the reaction : 2H3PO4(aq) + 3K2CO3 (aq)→ 3CO2 (g) + 3H2O(l) + 2K3PO4(aq) (c)Write the balanced complete ionic equation for the reaction (d)Write the balanced net ionic equation for the reactionarrow_forward2 Ag(s) + ¿ O2(g) + 2 H†(aq) 2 Ag*(aq) + H20(1) +1.23 O2(g) + 4 H*(aq) + 4 e¯ → 2 H2O(I) - +1.06 Br2(1) + 2 e¯ → 2 Br¯(aq) - +0.96 NO3¯(aq) + 4 H*(aq) + 3 e¯ NO(g) + 2 H2O(1) +0.80 Ag*(aq) + e-→ Ag(s) Now what are the values of E°, cell, AG°, and K?arrow_forwardConsider the reaction: Ba(NO 3) 2( aq) + Na 2SO 4( aq) → BaSO 4( s) + 2NaNO 3( aq) Which of the following statements is correct? Barium is oxidized. This reaction is not an oxidation-reduction reaction. Nitrate ion is the reducing agent. Sulfate ion is the oxidizing agent. Sodium is reduced.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning