
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
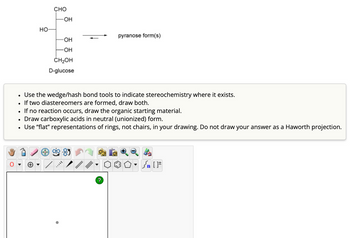
Transcribed Image Text:•
HO-
CHO
-OH
pyranose form(s)
-OH
-OH
CH₂OH
D-glucose
Use the wedge/hash bond tools to indicate stereochemistry where it exists.
If two diastereomers are formed, draw both.
If no reaction occurs, draw the organic starting material.
Draw carboxylic acids in neutral (unionized) form.
Use "flat" representations of rings, not chairs, in your drawing. Do not draw your answer as a Haworth projection.
{n [
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw the structure of the major organic product(s) of the reaction. You do not have to consider stereochemistry. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu.arrow_forwardDraw the product of the following compounds with the given image.arrow_forwardIs a fatty acid with the following structure saturated or unsaturated? CH3-CH,-CH, C-OH |3D Ωarrow_forward
- Organic Chemistryarrow_forwardQuestion 2 Which statement is consistent with the Hammond postulate? O the transition state of an endothermic reaction step structurally resembles the reactant. O it is used to assign the E/Z stereochemistry of alkenes. the less stable carbocation intermediate is formed in electrophilic additions to alkenes. O in additions of HX to alkenes, the H adds to the less substituted alkene carbon and the X adds to the more substituted alkene carbon. O the transition state of an exothermic reaction step structurally resembles the reactant. A Moving to another question will save this response. 80 F3 54 $ 4 000 000 F4 % 5 F5 6 MacBook Air F6 & 7 ao F7 8 DII F8 ( 9 F9arrow_forwardWhat is the correct answer and why?arrow_forward
- Is C-O ester of 3-chloro-4’-methoxychalcone conjugated (talking about the H3CO attatched to the benzene ring)? Can you explain your answer?arrow_forwardGive a brief answer to explain why acetic acid has a much higher BP (118 C) than 1- propanol (BP = 97 C) even though they both have the same size (molecular weight = 60) and they both form Hydrogen bonds.arrow_forwardDraw the structure of the major organic product obtained by hydroboration-oxidation of trans-3-methyl-3-hexene. H Click and drag to start drawing a structure. : ☐ G Parrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY