
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
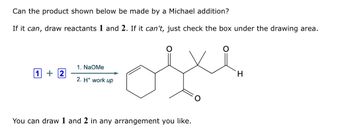
Transcribed Image Text:Can the product shown below be made by a Michael addition?
If it can, draw reactants 1 and 2. If it can't, just check the box under the drawing area.
1 +2
1. NaOMe
2. H+ work up
oste
You can draw 1 and 2 in any arrangement you like.
H
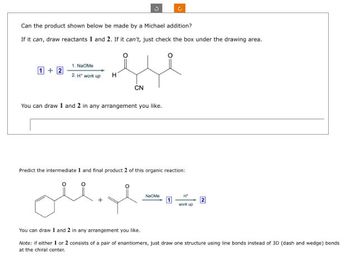
Transcribed Image Text:Can the product shown below be made by a Michael addition?
If it can, draw reactants 1 and 2. If it can't, just check the box under the drawing area.
1. NaOMe
1+2
2.H" work up
Η
CN
You can draw 1 and 2 in any arrangement you like.
Predict the intermediate 1 and final product 2 of this organic reaction:
Na Me
Ht
work up
You can draw 1 and 2 in any arrangement you like.
Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds
at the chiral center.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Use full-headed or half-headed curved arrows to show the movement ofelectrons in attached reaction.arrow_forwardFor the reaction below: a Draw the organic product(s). O AICI 3 • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ChemDoodleⓇ® Jn [1*arrow_forwardCan the product shown below be made by a Michael addition? If it can, draw reactants 1 and 2. If it can't, just check the box under the drawing area. 1. NaOMe +2 2. H+ work up CN You can draw 1 and 2 in any arrangement you like.arrow_forward
- Draw the product(s) of the reaction below. O: H + Add/Remove step Х Click and drag to start drawing a structure.arrow_forwardDraw the major product of the substitution reaction shown below. Ignore any inorganic byproducts. H₂O Draving CI Atoms, Bonds and Rings Charges Draw or tap a new bond to see suggestions. Undo Reset Remove Done Drag To Pan +arrow_forwardPRINTER VERSION 1 BACK NEXT Add curved arrow(s) to draw step 2 of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in this step. H,C CH, Close CH2 +. CI H.C CH2 Brarrow_forward
- Complete the mechanism for the keto-enol tautomerization shown using bonds, charges, nonbonding electron pairs, and curved arrows (forward reaction only). Do not delete any pre-drawn bonds, charges, or lone pairs. If you accidentally delete a vital part of the structure, click the undo button in the lower left. Step 1: keto form. Draw curved arrows. Step 2: complete the structure, then add curved arrows. Select Draw Rings More Erase Select Draw Rings More Erase H H - H :0: :0: :o: 1L :o:arrow_forwardDraw the product, please ก D L. CH,CH,CH,Mg Br 2. H₂O 1. H₂O* 2. PCC J. CH,MgBr 4. HO LHCN KEN 2. H,O Aarrow_forwardConsider the transformation shown below. Br HO Br i. Calculate the oxidation number for the carbon atom that experiences changes in its bonds. Nox(starting material) = Nox(product) = ii. Determine whether an oxidation, reduction, or neither took place. Answer oxidation or reduction or neither: iii. Determine the number of electrons transferred. Answer should be a positive integer: 1 iv. Provide the reagent(s) that are needed to produce the transformation shown. Reagents should be listed in alphabetical order (numbers come before letters) and separated by commas. Do not use subscripts. (Use the Reagent Cabinet List as a reference for correct formatting of your answer.) Reagents:arrow_forward
- For the mechanism, draw the curved arrows as needed. Include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly in your products. Do not use abbreviations such as Me or Ph. Step 1 NaOH, H₂O Heat + i Incorrect. Which of the mechanistic steps is shown in this step of the mechanism? Draw step 1 of the mechanism. CH3 CH3 + Edit Drawingarrow_forwardneed answer fast i'll rate uparrow_forwardNonearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY