
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
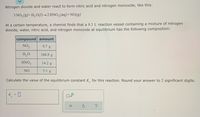
Nitrogen dioxide and water react to form nitric acid and nitrogen monoxide, like this:
3 NO2 (g) H20(l) 2 HN03(aq) + NO(g)
At a certain temperature, a chemist finds that a 8.3 L reaction vessel containing a mixture of nitrogen
dioxide, water, nitric acid, and nitrogen monoxide at equilibrium has the following composition:
compound amount
NO2 9.7 g
H2O 104.8 g
HNO3 14.2 g
NO 5.1 g
Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits.
Transcribed Image Text:Nitrogen dioxide and water react to form nitric acid and nitrogen monoxide, like this:
3 NO, (g)+H, O(1)→2 HNO3(aq)+NO(g)
At a certain temperature, a chemist finds that a 8.3 L reaction vessel containing a mixture of nitrogen
dioxide, water, nitric acid, and nitrogen monoxide at equilibrium has the following composition:
compound amount
NO,
9.7 g
H,0
104.8 g
HNO3
14.2 g
NO
5.1 g
Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chlorine and water react to form hydrogen chloride and oxygen, like this: 2 Cl₂(g) + 2H₂O(g) → 4 HCl(g) + O₂(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound pressure at equilibrium C1₂ 2.75 atm H₂O HC1 02 Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K = 4,-0 33.5 atm 24.2 atm 62.0 atm ☐ x10 ?arrow_forwardMethanol and oxygen react to form carbon dioxide and water, like this: 2 CH,OH(1)+3 O2(9)→2CO,(9)+4 H,0(g) At a certain temperature, a chemist finds that a 9.6 L reaction vessel containing a mixture of methanol, oxygen, carbon dioxide, and water at equilibrium has the following composition: compound amount CH;OH 1.31 g O2 2.86 g CO2 2.88 g H,0 2.43 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ = 0 x10arrow_forwardIron(III) oxide and hydrogen react to form iron and water, like this: Fe,O3(s)+3 H2(9)–→2 Fe(s)+3 H,O(g) At a certain temperature, a chemist finds that a 4.5 L reaction vessel containing a mixture of iron(III) oxide, hydrogen, iron, and water at equilibrium has the following composition: compound amount Fe,O3 3.54 g H2 3.20 g Fe 4.49 g H,O 1.06 g Calculate the value of the equilibrium constant K_ for this reaction. Round your answer to 2 significant digits.arrow_forward
- Titanium(IV) chloride decomposes to form titanium and chlorine, like this: TİCL,(1)→Ti(s)+2 Cl,(9) At a certain temperature, a chemist finds that a 3.4 L reaction vessel containing a mixture of titanium(IV) chloride, titanium, and chlorine at equilibrium has the following composition: compound amount TiCl, 3.86 g Ti 2.68 g Cl2 1.40 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ = ] x10arrow_forward4 Titanium(IV) chloride decomposes to form titanium and chlorine, like this: Ticl,()-Ti(s)+2 Cl,(9) At a certain temperature, a chemist finds that a 7.7 L reaction vessel containing a mixture of titanium(IV) chloride, titanium, and chlorine at equilibrium has the following composition: compound amount TiCl, 4.90 g Ti 1.24 g Cl, 2.16 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K = 0 Submit Assignment Continue O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility Finder F12 F11 F9 F10 F8 F7 F5 F6 .arrow_forwardMercury and oxygen react to form mercury(II) oxide, like this: 2 Hg(1)+O2(g)–→2 HgO(s) At a certain temperature, a chemist finds that a 6.3 L reaction vessel containing a mixture of mercury, oxygen, and mercury(II) oxide at equilibrium has the following composition: compound amount Hg 5.8 g O2 22.5 g HgO 18.9 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ = 0arrow_forward
- Mercury and oxygen react to form mercury(II) oxide, like this: 2 Hg(1)+O2(g)→2 HgO(5) At a certain temperature, a chemist finds that a 3.6 L reaction vessel containing a mixture of mercury, oxygen, and mercury(II) oxide at equilibrium has the following composition: compound amount Hg 9.7 g O2 21.8 g HgO 10.2 g Calculate the value of the equilibrium constant K̟ for this reaction. Round your answer to 2 significant digits. K_ = 0arrow_forwardMercury and oxygen react to form mercury(II) oxide, like this: 2 Hg(1)+O2(g)→2 HgO(s) At a certain temperature, a chemist finds that a 7.0 L reaction vessel containing a mixture of mercury, oxygen, and mercury(II) oxide at equilibrium has the following composition: compound amount Hg 7.0 g O2 5.8 g HgO 16.5 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ = | x10arrow_forwardNitrogen dioxide and water react to form nitric acid and nitrogen monoxide, like this: 3 NO₂(g) + H₂O(1)→2 HNO3(aq) + NO(g) At a certain temperature, a chemist finds that a 3.4 L reaction vessel containing a mixture of nitrogen dioxide, water, nitric acid, and nitrogen monoxide at equilibrium has the following composition: compound NO₂ H₂O HNO3 NO amount 5.9 g 142.0 g 15.1 g 12.0 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. с K = D 0 C x10 X Śarrow_forward
- Mercury and oxygen react to form mercury(II) oxide, like this: 2 Hg(1)+O2(9)→2 HgO(s) At a certain temperature, a chemist finds that a 10. L reaction vessel containing a mixture of mercury, oxygen, and mercury(II) oxide at equilibrium has the following composition: compound amount Hg 12.7 g O2 13.4 g HgO 15.5 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K = | Submit Assignm Continue O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center| Access 43.203 FEB 19 MacBook Airarrow_forwardThe equilibrium constant, Kc , for the following reaction is 7.00×10-5 at 673 K.NH4I(s) NH3(g) + HI(g)If an equilibrium mixture of the three compounds in a 4.05 L container at 673 K contains 2.71 mol of NH4I(s) and 0.262 mol of NH3, the number of moles of HI present is moles. --- The equilibrium constant, Kc, for the following reaction is 6.30 at 723K.2NH3(g) N2(g) + 3H2(g)If an equilibrium mixture of the three gases in a 12.1 L container at 723K contains 0.250 mol of NH3(g) and 0.208 mol of N2, the equilibrium concentration of H2 is M.arrow_forwardIron and water react to form iron(III) oxide and hydrogen, like this: 2 Fe(s) + 3 H₂O(g) → Fe₂O3(s)+ 3 H₂(g) At a certain temperature, a chemist finds that a 2.5 L reaction vessel containing a mixture of iron, water, iron(III) oxide, and hydrogen at equilibrium has the following composition: compound Fe H₂O Fe₂O3 H₂ amount 4.04 g 2.87 g 3.49 g 2.08 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. C K = 0 C Dx1 x10 X Śarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY