
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I need full detail and accurate answer with full details.
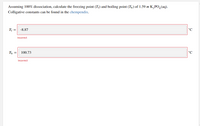
Transcribed Image Text:Assuming 100% dissociation, calculate the freezing point (T;) and boiling point (T,) of 1.59 m K,PO,(aq).
Colligative constants can be found in the chempendix.
Tf =
-8.87
°C
Incorrect
Th =
100.73
°C
Incorrect

Transcribed Image Text:amacmillan
learning
Colligative Constants
Chempendix
Constants for freezing-point depression and boiling-point elevation calculations at 1 atm:
Physical Constants
Kf value* Normal freezing K, value Normal boiling
(°C/ m)
Acids and Bases
Solvent
Formula
(°C/ m)
point (°C)
point (°C)
H20
C 6 H6
cyclohexane C 6 H 12
C2H60 1.99
water
1.86
0.00
0.512
100.00
Activity Coefficients
benzene
5.12
5.49
2.53
80.1
Activity Series
20.8
6.59
2.92
80.7
ethanol
-117.3
1.22
78.4
Amino Acids
carbon
|CCI 4
29.8
-22.9
5.03
76.8
tetrachloride
Boiling Points
camphor
С 10 Н 16 0 37.8
176
Bond Energies
Bond Lengths
Codons
*When using positive Kf values, assume that ATf is the absolute value of the change in temperature. If you would prefer to define ATfas "final minus initial"
temperature, then ATf will be negative and so you must use negative Kf values. Either way, the freezing point of the solution should be lower than that of
Colligative Constants
the pure solvent.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- hrome File Edit View History Bookmarks Profiles Window Help A 34% O Tab Mon O Ask Laftan Anlamaz - Episode x O st. John's University - My Ap x A ALEKS - Iffat Khan - Learn D YouTube G whats an iv - Google Search www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNsIkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGfE9IMFjvvh3x_rz6naF9YTN80Bms6y067EgyeOUwHKJOrzx. O STOICHIOMETRY Using molarity to find solute moles and solution volume Calculate the volume in milliliters of a 0.731 mol/L sodium nitrate solution that contains 150. mmol of sodium nitrate (NaNo,). Round your answer to 3 significant digits. のarrow_forwardhow to find average density and standard deviarrow_forwardComplete the percent errorarrow_forward
- Chrome File Edit View History Bookmarks Profiles Window Help Tab 21% D BC Broward College | Affordable x O Onelogin 1A BC - Student Registration O Launch Meeting - Zoom ALEKS - Esther Octave - Le i www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H2oW_5PTMi0acVC-XJc4zwaxK8Wf2BSBxnL8YsAgEaj1JZm27.. * E Apps M Gmail O YouTube Maps BC Broward College |. O Sample Source An... O New Tab G What does Duckw.. E Untitled documen. O CHEMICAL REACTIONS Calculating the heat of reaction from molar reaction enthalpy a... Esth A chemist measures the energy change AH during the following reaction: 2 Fe,O3(s) → 4 FeO(s)+O2(g) AH=560. kJ Use the information to answer the following questions. O endothermic. This reaction is... O exothermic. Suppose 27.6 g of Fe,O, react. O Yes, absorbed. O Yes, released. Will any heat be released or absorbed? O No. If you said heat will the second part of this question, calculate how much heat will be released or absorbed. released or absorbed…arrow_forwardBa(NO,l(aq) + K,SOlaq) Baso(s) + 2 KNOlaq) Volume Volume Trial 0.10 M Ba(NO)2 (ml) 0.10 M K,SO, (ml) Mass Baso4 (s) Limiting Reagent 50. 20. 0.46 50. 40. 0.92 3 50. 50. 1.15 4 50. 60. 1.15 50. 80. 1.15 • Question 8 Use the data to identify the limiting reagent for each reaction performed. V Trail 1 a. Neither/Both EV Trial 2 b. Baso4 EV Trial 3 C. KSO4 EV Trial 4 d. Ba(NO3)2 EV Trial 5 e. KNO3 Question Help: Message instructor I Calculator Submit Question • Question 9 Why is the amount of precipitate in the Data Table above the same in Trials 3 through 5? Edit Insert Formats B IVX x' Aarrow_forward92% D Sun 3:04 PM OneLogin B McGraw-Hill Can O Question 1- 8.4 M MHE Reader B McGraw-Hill Cam x A ALEKS - Esther C x -> A www-awa.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H2oW_5PTMiOacVC-TPIQJ5aqZ8Tg8iSQs6Rzj49leNgLwH_Jfre.. ☆ -pps M Gmail O YouTube O Maps BC Broward College |.. O Sample Source An.. O New Tab G What does Duckw. E Untitled documen. B Reading List O KINETICS AND EQUILIBRIUM Using reactant reaction order to predict changes in initial rate Esther A certain reaction is first order in H, and first order in I,. Use this information to complete the table below. Be sure each of your answer entries has the correct number of significant digits. [4] [1] initial rate of reaction 1.25 M 1.39 M 15.0 M/s do 0.431 M 1.39 M 2.06 M 0.844 M Check Explanation O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy | Accessibility 642 4 MacBook Airarrow_forward
- Course Contents » ... Timer Notes Evaluate Feedback Print Inf 3. What type of laboratory glassware is used when the accuracy and precision of volume is critical to quantitative analysis? Enter the one word term, using lower case letters, that represents this type of glassware. hmit Answer Tries O /99arrow_forwardI asked this before and the person said there was no data....i submitted the data in one picture and question in the second image! this is literally all of the information i have! that's why i am asking for help!!!arrow_forwardGive typed full explanation not a single word hand written otherwise leave itarrow_forward
- 100% Реople Tab Window Help File Edit View History Bookmarks Chrome Q Solutions and Interm x Q Chemistry- Solution x Answered: What is 101 Chem101 Q C16 Chemistry Ch16 x ... B Lecture Chapter 9 -> i app.101edu.co O YouTube M Gmall A Maps B #14 CRM Study B.. A (16) Piggy (ALPH. E Apps American Express.. ud Urban Dictionary:. O News Translate Question 16 of 35 Convert the concentration of 0.700 M Na,so, to g/mL X STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 83.3 142.04 0.0994 6.022 x 1023 1000 1 119.05 99.4 0.700 0.001 0.0833 mL M Na,SO, g Na* mol Na* g/mL mol NazSO, g Nazso, 回■O區民@T山 MacBook Pro #3 1 3 4. & 6. 7. 8. Q W Tarrow_forwardPls do fast and i will like for sure Try to give solution in typed form.. Given is the mean and Average deviation. There is no standard deviation given, How are you able to get percent relative standard deviation with out it?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY