
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ome File Edit View History Bookmarks Profiles
Chai
=
G numb G aceto
Cell t b Answb Answ My DA AL x CA stur CA sol
C www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInMX9hNGVYTIJ JZmF5TTEgJckvK3y58Hg
Solubility and... 18.3 Gibbs Free E... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... SOLUTION: The le... Math 115 W-S Fall...
(51)
OSTATES OF MATTER
Calculating molality
Micro To do Neuro
& list of
□
esc
molarity =
molality = 0
Explanation
1
Q
A
2
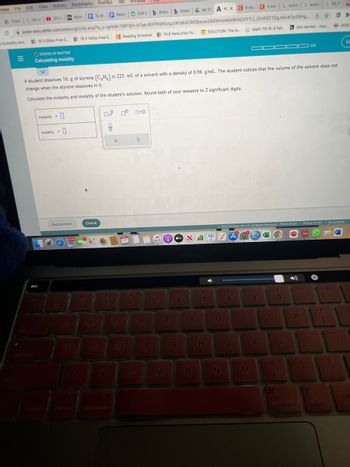
A student dissolves 10. g of styrene (CH₂) in 225. mL of a solvent with a density of 0.96 g/mL. The student notices that the volume of the solvent does not
change when the styrene dissolves in it.
Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.
Z
Check
W
Tab
S
8
#
3
X
Window
E
0° 0x0
17
D
$
4
C
5
R
F
%
5
V
tv
A
6
T | Y
G
B
&
7
H
CE
U
N
8
—
J
M
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
97 A
9
▬▬
O
KL
<
1
0
H
Get started.: Hyp
P
0 ☆
command
0/5
Aa
G 96/1
alt
..
option
2020
La
(
80
1
Expert Solution
arrow_forward
Step 1
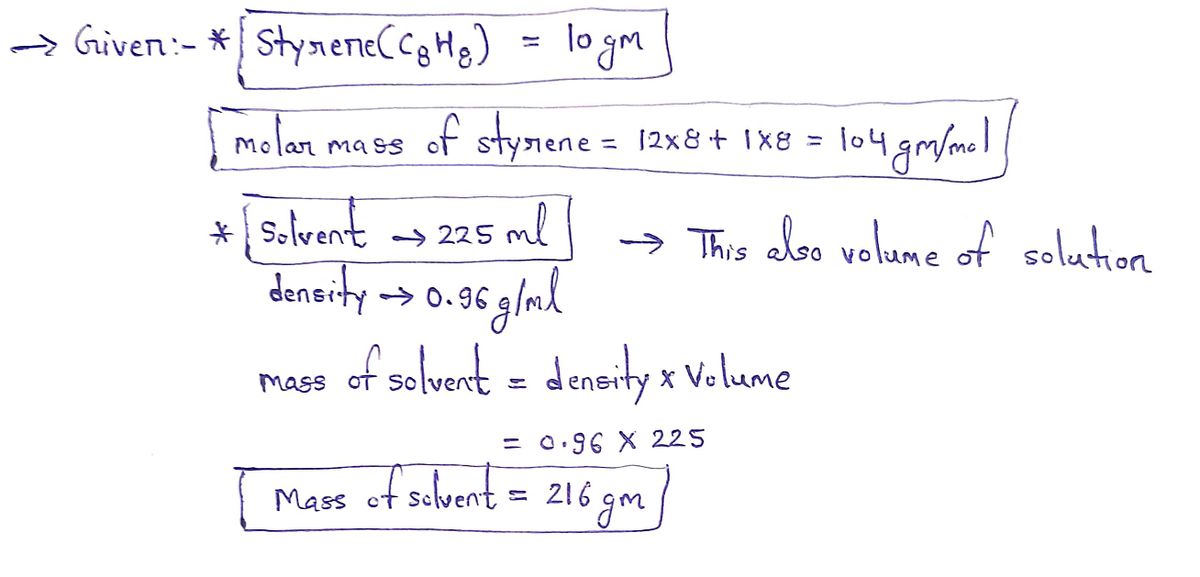
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume of a concentrated HCl solution, which is 36.0% HCl by mass and has a density of 1.179 g/mL, should be used to make 5.30 L of an HCl solution with a p of 1.90? Express your answer to two significant figures and include the appropriate units. a) ? HA Value Unitsarrow_forwardThe mass of solute per 100 mL of solution is abbreviated as (m/v). Mass is not technically the same thing as weight, but the abbreviation (w/v) is also common. How many grams of sucrose are needed to make 705 mL of a 39.0% (w/v) sucrose solution? mass: g sucrosearrow_forwardHow many grams of water must be used to prepare a 2.50 percent by mass (%m/m) sodium hydroxide (NaOH) solution from 12.7 g NaOH? Report your result in decimal notation and to the proper number of significant figures. All numbers are measured. a) 2.50 g water b) 5.08 g water c) 12.7 g water d) 495 g water e) 508 g waterarrow_forward
- A chemist prepares a solution of barium chloride BaCl2 by measuring out 44.0μmol of barium chloride into a 150.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's barium chloride solution. Round your answer to 3 significant digits.arrow_forwardIsopropyl alcohol is mixed with water to produce a solution that is 33.0%33.0% alcohol by volume. How many milliliters of each component are present in 895 mL895 mL of this solution?arrow_forwardCalculate the volume in milliliters of a 0.448M potassium iodide solution that contains 150.mmol of potassium iodide KI. Be sure your answer has the correct number of significant digits.arrow_forward
- 1. A 15.00 g of NaCI is dissolved in 5.00 L of water. Find the molarity of the solution? Show yoursolutions for MW. (Use whole numbers for the MM and 2 decimal places for the Final Answer)arrow_forwardA chemist prepares a solution of barium chloride BaCl2 by measuring out 88.μmol of barium chloride into a 300.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /molL of the chemist's barium chloride solution. Be sure your answer has the correct number of significant digits. molLarrow_forward1 g of a 404.7 % NaCl solution is administered to a patient. How many grams of NaCl did the patient receive? Report your answer to 2 significant figures.arrow_forward
- A chemistry student needs 15.0 g of heptane for an experiment. He has available 0.50 kg of a 44.3% w/w solution of heptane in diethyl ether. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to 3 significant digits. g x10 No solutionarrow_forwardYou have a solution of NaCl that has a molarity of 3.359 M. You want to make a dilute solution of NaCl that has a molarity of 0.955 M and you need that dilute solution to have a final total volume of 808.76 mls. How many mls of the concentrated solution will you use to make that desired dilute solution? Answer must NOT contain units. Use proper significant figures.arrow_forwardA 125ml sample of an 8.2 M NaCl solution is diluted to 3.0 L . What volume of the diluted solution contains 10.8 g of NaCl ? Express your answer using two significant figures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY