
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
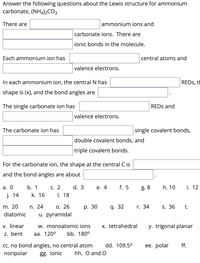
Transcribed Image Text:Answer the following questions about the Lewis structure for ammonium
carbonate, (NHa)½CO3
There are
ammonium ions and
carbonate ions. There are
ionic bonds in the molecule.
Each ammonium ion has
central atoms and
valence electrons.
In each ammonium ion, the central N has
REDS, tH
shape is (x), and the bond angles are
The single carbonate ion has
REDS and
valence electrons.
The carbonate ion has
single covalent bonds,
double covalent bonds, and
triple covalent bonds.
For the carbonate ion, the shape at the central C is
and the bond angles are about
а. О
b. 1
С. 2
d. 3
е. 4
f. 5
g. 8
h. 10
i. 12
j. 14
k. 16
I. 18
m. 20
n. 24
О. 26
р. 30
q. 32
r. 34
S. 36
t.
diatomic
u. pyramidal
v. linear
z. bent
w. monoatomic ions
x. tetrahedral
y. trigonal planar
aа. 1200
bb. 180°
ff.
c. no bond angles, no central atom
nonpolar
dd. 109.5°
ее. polar
gg. ionic
hh. O and O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7.30 The bond in HF is said to be polar, with the hydrogen carrying a partial positive charge. For this to be true, the hydrogen atom must have less than one electron around it. Yet the Lewis dot structure of HF attributes two electrons to hydrogen. Draw a picture of the electron density distribution for HF and use it to describe how the hydrogen atom can carry a partial positive charge. How can these two models of the HF bond (the electron density and the Lewis structure) seem so different and yet describe the same thing?arrow_forwardWhich of the following statements is false concerning bonding? Elements with extremely different electronegativities tend to form ionic bonds with each other. In an N—O bond. electron density is greater near the O atom. An N—O bond is an example of a polar covalent band. In general, chemical bonds form to minimize energy. The bond in KBr is formed by sharing electrons.arrow_forward7.50 Chemical species are said to be isoelectronic if they have the same Lewis structure (regardless of charge). Consider these ions and write a Lewis structure for a neutral molecule that is isoelectronic with each of them, (a) CN , (b) NH4+ . (c) CO32arrow_forward
- In the hypervalent ion ICl4-, the central I atom forms single bonds to each of the 4 Cl atoms. How many non-bonding electrons are in the sphere of the centra I atom?arrow_forward101 107 317DOW 3 Draw a Lewis structure for NH 3 in which the central N Atom obeys the octet rulle, and answer the following questions. O waist auto Ch 0 The number of unshared paies (lone pairs) on the centent Natomis: opxo to slam? The central Natoms forms The central N atoms forms double bonds. single bondsin LUE OCULarrow_forwardIn the hypervalent ion ICl3-, the central iodine atom forms single bonds to each of the 3 Cl atoms. How many non-bonding electrons are in the sphere of the central iodine atom?arrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D H - H H H I C chemical symbol, chemical formula or Lewis structure — - H I C - Z : N 1 C 1 H - H H H - - Ar 0. H 1 CIO Cr · Η .. - H H 1 H ▪ H boiling point ✓ (Choose one) 1 (highest) 2 3 4 (lowest) (Choose one) V (Choose one) ✓ (Choose one) varrow_forwardAnswer the questions in the table below about the shape of the phosphorus pentabromide (PBr,) molecule. How many electron groups are around the central phosphorus atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central phosphorus atom? (You may need to use the scrollbar to see all the choices.) (choose one) Ar Explanation Check © 2021 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center | Accessibilityarrow_forwardWrite the Lewis structure for each molecule or ion. Include resonance structures ifnecessary. Then assign the electron geometry, molecular geometry, and bond angles CN-arrow_forward
- anion. Answer the questions in the table below about the shape of the orthotellurate (Teo-) a How many electron groups are around the central tellurium atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central tellurium atom? (You may need to use the scrollbar to see all the choices.) 山 (choose one) X Sarrow_forwardCorrect the following statement: “The bonds in solid PbCl2 are ionic; the bond in a HCl molecule is covalent. Thus, all of the valence electrons in PbCl2 are located on the Cl– ions, and all of the valence electrons in a HCl molecule are shared between the H and Cl atoms.”arrow_forwardBased on Lewis structures, predict the ordering, from shortestto longest, of N¬O bond lengths in NO+, NO2-, andNO3-.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning