
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Oo.10.
Subject:- Chemistry
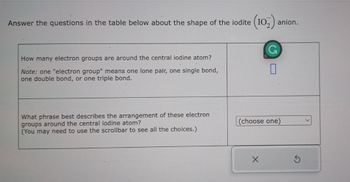
Transcribed Image Text:Answer the questions in the table below about the shape of the iodite (102) anion.
How many electron groups are around the central iodine atom?
Note: one "electron group" means one lone pair, one single bond,
one double bond, or one triple bond.
What phrase best describes the arrangement of these electron
groups around the central iodine atom?
(You may need to use the scrollbar to see all the choices.)
0
(choose one)
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to Wikipedia, Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon. The method was developed in the late 1940s at the University of Chicago by Willard Libby, who received the Nobel Prize in Chemistry for his work in 1960. It is based on the fact that carbon- 14 is constantly being created in the atmosphere by the interaction of cosmic rays with atmospheric nitrogen. The resulting carbon-14 combines with atmospheric oxygen to form radioactive carbon dioxide, which is incorporated into plants by photosynthesis; animals then acquire carbon-14 by eating the plants. When the animal or plant dies, it stops exchanging carbon with its environment, and thereafter the amount of carbon-14 it contains begins to decrease as the carbon-14 undergoes radioactive decay. Measuring the amount of carbon-14 in a sample from a…arrow_forwardThe Kyp of Cul is 1.1 x 10-12 Part A Find Ecell for the cel: Cu(s) | CuI(s) |I-(aq, 0.90 mol L-1) || Cu* (aq, 0.90 mol L-1)| Cu(s) Express your answer to two significant figures and include the appropriate units. ? Ecell = Varrow_forward10:31 PM Tue Oct 12 AA www-awn.aleks.com G periodic table - Goo... Favorites - YouTube... XA ALEKS - Caliyah Hel... G kno3 acid or base -... G linear programming... G write formulas for t... C Solved A. Objective Knowledge Check Question 6 Caliyah A chemistry student weighs out 0.259 g of ascorbic acid (H,C,H,O,), a diprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1400M NAOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Be sure your answer has the correct number of significant digits. mL x10 Privacy Center Accessibilityarrow_forward
- Emerson M Cremistr pdated late workE a educate.lindsay.k12.ca.us/iFrame.aspx?iCtri=STUDENT BASE HOME CONTROL O Empower 2021 O Ermpower 2021 pokmarks DE IXL Classes O Google Slides O Empower 2020 A Google Docs t Lindsay High School Physics Safety & Ski. E Guided pract 2-1 googlegalaxysclence.com Li Be 1-0 1-5 2-0 Al 2.5 3-0 3-5 Na Mg 1-2 Si S CI 0-9 1-5 1-8 Ge 2-1 2-5 3-0 K Ca Ga 1-6 As Se Br 0-8 1-0 1-8 2-0 2-4 Te 2-8 Rb Sr In Sn Sb 0-8 1-0 1-7 1-8 Pb 1-9 2-1 2-5 Cs 0-7 Ba TI Bi Po At 2-2 Review the chart above. It is showing how much energy it takes to remove an electron from each of those types of atoms. Why would 0.9 1-8 1-9 1-9 2-0 it be so high in the top right comer and so low in the lower len comer? It would be high in the top right coner because these electrons are closer to the nucleus and held more strongly, so they will take O A. more energy to remove. And the opposite is true for the lower left, farther from the nucleus and so not held as strongly, requiring less energy to…arrow_forwardCaps Tab Wall wen M Inbox (1.600)-ftantiudeledux Mail-Francesca A Tantillo-Out xEXP #12: Geometry-CHM150-2 x Aktiv Chemistry ← → C app.101edu.co 90°F Mostly sunny 1 D Q Z Uranium hexafluoride, UF, is an important compound used in the enrichment of uranium by gaseous diffusion. A A Graham's Law states that rate of effusion A/rate of effusion of B = (square root of Mass of B)/(square root of Mass of A). Calculate how fast 235 UF gas diffuses compared to 230UF. gas. Assume the temperature remains constant. 2 W S 3 E D # C $ 4 R OL F C ▷ll s FS % 5 T V C Question 19.c of 23 G P A 6 Y B H & 7 PrtScn N J 8 N Home 1 M ( 9 K End o O < 1 4 7 +/- PgUp 0 L times faster than 238UF 2 5 8 12 4 P 3 6 9 0 □ A PED PgOn 1924 G + 0 Update Submit C x 100 BE 5:22 PM 7/6/2022 Del Backspace 과arrow_forwardIs the final answer correct ? Or would there only be two significant figures in the final answer ?arrow_forward
- Please answers number 1-4 along with the with the correct solving of how you got the answers, I'm still confused on these.arrow_forwardHow do I get the mass of Cu, g produced?arrow_forwardMany common materials that we ingest, though quite safe in reasonable quantities, become toxic when taken in very large doses. A measure of toxicity is the LD50 value (Lethal Dose, 50%). It is the quantity of material, expressed in mg of material per kg of subject-body-weight that, if administered to a population of subjects, would cause 50% of the population to die. The LD50 value for FD&C Red Dye No. 40 is >10,000 mg/kg in rats. Assume that the LD50 value for humans is the same as for rats. Calculate the number of mg of Allura Red present in an 8 fluid ounce glass of the beverage you used in this lab. Assume that the concentration of Allura Red in the beverage is 0.000054 M. The molar mass of Allura Red is 496.42 grams/mol 1 fl oz = 29.5735 mL Do not include units with your answer.arrow_forward
- A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IJgXZp57itWHhRgilODc5Mqv... G Apps CSU Study/Teach Abro... GACE Testing O Degree Related E E-Tandem E Grade Calculator 国Re Korean O STATES OF MATTER Identifying the intermolecular forces between atoms, ions and... Kyli. What kind of intermolecular forces act between a bromine (Br,) molecule and a tetrachloroethylene (C,Cl4) molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.arrow_forward2 Automobile air bags inflate following a serious impact. The impact triggers the following chemical reaction. 2NaN3 (8)→ 2 Na(s) + 3N₂ (9) S W stry.com/myct/itemView?assignmentProblemID=213377858&attemptNo=2&offset=next X 7 # 3 E D 80 C $ 4 R 888 F V % 5 FS T Y G A Part A 6 B If an automobile air bag has a volume of 11.2 L, what mass of NaN3 (in g) is required to fully inflate the air bag upon impact? Assume STP conditions. IVE ΑΣΦ Provide Feedback m = Submit MacBook Air F6 Y H & 7 Request Answer N 40 U J * 8 @ C PwC FB I M ( 9 K DD ? O ) O g L command 3 F10 P . : ; Review I Constants I Periodic Table I { + [ option = 11 ? 1 I 323) Next > 1 deletearrow_forwardL, Of the container from part 'c'. ILS) A 0.50-kilogram serving of milk has an energy content of 371 kcal. A 0.50-kilogram uVing of ground beef has an energy content of 1470 kcal. A 60.00-kilogram man drinks 0.50 kilograms of milk in two minutes. At the same time, his 60.00-kilogram identical twin brother eats 0.50 kilograms of ground beef in two minutes. Immediately after the food is consumed, is one brother heavier than the other? If so, identify the heavier brother and explain why he is heavier. If not, explain why neither brother is heavier.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY