
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please solve it
Transcribed Image Text:O-E
PAS
(a)
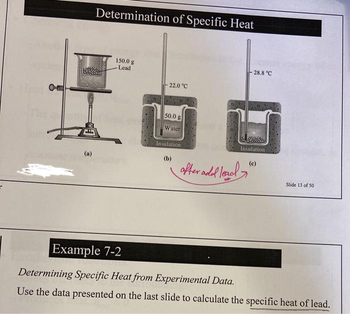
Determination of Specific Heat
150.0 g
Lead
- 22.0 °C
50.0 g
Water
Insulation
(b)
after add lead
28.8 °C
Insulation
(c)
Slide 13 of 50
Example 7-2
Determining Specific Heat from Experimental Data.
Use the data presented on the last slide to calculate the specific heat of lead.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which inventions resulted from applying Charles law?arrow_forward1. Now you will mass out a sample of the sodium bicarbonate. You will need to open the bottle by pointing at the lid and it will say Remove Lid. Next, grab the scoop and drag it to the open mouth of the bottle. Then, as you move the scoop down the label of the bottle, it will fill up to different amounts. Select the largest amount and drag the scoop to the weigh paper in the balance and let the sample go. This should be approximately 1 gram of sample on the balance. Add a second scoop to the weigh paper so that you have approximately 2 grams of sodium bicarbonate total. Be sure to record the total mass, to the correct significant figures. You will find a data table in this procedure. Drag the weigh paper and your sample to the beaker and release the sample. It is now in the beaker and you are ready to proceed. Click the green Zoom Out arrow 2. Now it is time to make your solution. Generally, chemists would use a volumetric flask for this, but that is not available in this…arrow_forwardCan you show me the calculations please ? I'm not following how you got to this number ?arrow_forward
- I have calculated this problem various times, but every time I input the answer into the assignment it says it's wrong, I can't find the issue. What could it be?arrow_forward23. What will happen if an inflated balloon is placed in front of a floor register 23. that blows a warm air? A. The balloon will get completely deflated B. The balloon will become larger. C. The balloon will become smaller. D. The balloon will maintain its size. E. All the above will happen.arrow_forwardprovide complete solutionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY