
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please solve the problem as soon as possible .
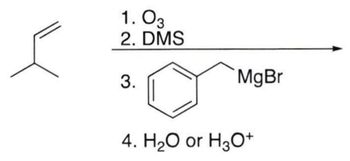
Transcribed Image Text:1.03
2. DMS
3.
MgBr
4. H₂O or H3O+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Here is a graph of the molarity of formic acid (HCO₂H) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. M 0.030- 0.025 0.020 0.0187 0.015 0.010 0.005 0 y 500 1000 1500 seconds Is HCO₂H being created or destroyed by the chemical 2 reaction? 2000 O 2500 created destroyed 3000 X neither created nor destroyed x10 010 X Śarrow_forwardWhich among the following must be satisfied for a collision between particles to occur? Particles of reacting substances must have the same kinetic energy and speed The particles of reacting substances must collide with a particle they are reactive to Both particles of reacting substances must be the same size and shape The orientation of a substance must be appropriate for the occurring reactionarrow_forwardanswer all parts to the question please!arrow_forward
- Help me fill out the rest please.arrow_forwardGo Figure 14.7 160 140 120 100 80 60 40 20 10,000 20,000 30,000 Time (s) (a) In pressure, CH₂NC 5.2 5.0 4.8 4.6 4.2 4.0 3.8 3.6 3.4 0 10,000 20,000 Time (s) (b) Kinetic data for conversion of methyl isonitrile into acetonitrile. 30,000 Part A Why is the slope of the line in part (b) negative? Match the words in the left column to the appropriate blanks in the sentences on the right. Submit decreases first-order k zero-order remains the same same negative -k positive increases second-order Request Answer This is a reaction is Review | Constants | Periodic Table Reset reaction, therefore, a graph of the natural logarithm of the pressure versus time is a straight line. The slope of the line in part (b) is negative because the amount of methyl isonitrile Because the slope of the line equals the rate constant for this Help 10 of 36arrow_forward0.003 0.00243 0.002 M 0.001 5 10 15 20 25 30 seconds created Is C,H,OH being created or destroyed by the chemical destroyed reaction? neither created nor destroyed If C,H,OH is being created or destroyed, what is the rate at which it is being created or destroyed 13 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If C,H,OH is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 13 seconds of the reaction? O Oarrow_forward
- I need help with my chemistry homeworkarrow_forwardvery strong back drugs on the tumor sizes of mice. The doctor is searching for the correct combination of medications that will make the tumor shrink in size. The drugs he has at his disposal are drugs / B and C. His experiments are summarized below. Average rate of Exp. [A), in [B], in [C], in shrinkage M M M # |(mm/week) 0.0010 0.0020 0.0030 0.010 II 0.0010 0.0010 0.0020 0.0050 0.0030 0.0020 0.0015 0.090 0.0030 0.0020 0.0030 0.090 II IV What is the rate law for this system? %23arrow_forwardI only need question 4,arrow_forward
- Here is a graph of the pressure of nitrogen dioxide (NO,) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. 30- 25 - 20- 18.707 atm 15- 10- 10 15 20 25 30 seconds O created x10 Is NO, being created or destroyed by the chemical destroyed reaction? neither created nor destroyed If NO, is being created or destroyed, what is the rate at which it is being created or destroyed 9 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If NO, is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 9 seconds of the reaction? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardWhat is method of initial rates and what does it do?arrow_forward2. The decomposition reaction of HO2 is given by 4 HO, (g) → 2 H2O (g) + 3 O, (g). From the following kinetics data (concentration of HOz vs. time) and two figures below, answer the following questions. Time (min) [HO;] M (or mol/L) 0.0000 2.0000 1.2500 0.66667 0.5 2.5000 0.40000 3.7500 0.28571 In [ HO, ] 1 5.0000 0.22222 [ HO, 1 4.5 2. The reaction order of HO2 should -1 be order. 1.5 a) Zero-th b) First c) Second time (min) time (min) d) None of the abovearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY