
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
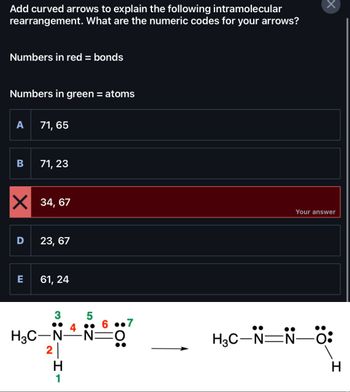
Transcribed Image Text:Add curved arrows to explain the following intramolecular
rearrangement. What are the numeric codes for your arrows?
Numbers in red = bonds
Numbers in green = atoms
A
B
D
71, 65
X 34,67
E
71, 23
23, 67
61, 24
3
H3C-N
2
H
1
5
4.
6.7
·N=O
Your answer
N—Ö:
H₂C–N=N-
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw all possible reasonable structures for the following. For each molecule if any appears significantly less stable , circle it . Solve 1, 2, 3,4arrow_forward4&6 are attached questions, thank you!arrow_forwardThese are the resonance structures of pyridine. Why are the electrons given to the nitrogen in the first move and not to the b bond or the adjacent carbon as a lone pair? How do I know where the first move will be and how many moves I will make (1 or 2) in such cases where I have circular structures? 5 :N - S Ć :Z:arrow_forward
- May you please help me with this ochem question?arrow_forward2. Provide the missing curved electron arrows for the reaction below. H нн CH3 + H.о + NаBr NaOH CH3 Jorarrow_forwardCopy the figure shown below onto your scratch paper. Вох 1 Na Вох 2 Na® SCH3 Br Br SCH3 Then, complete the figure on your scratch paper by drawing out the intermediates and arrow-pushing mechanism in Boxes 1-2 that explain the formation of the final products shown. You should start your arrow-pushing in Box 1, and you will use all boxes. Now, report the formal charges (+,0,-) that appear on the sulfur, bromine, and alpha carbon atoms in each box, and match the contents of each box to a single phrase (A-G) that best describes the mechanistic step that occurs in each box. A. A nucleophile attacks a tertiary carbocation. B. A nucleophile attacks a secondary carbocation. C. A nucleophile attacks, and a leaving group leaves. D. Br is protonated, and S is deprotonated. E. A leaving group leaves. F.S is protonated, and Br is deprotonated. G. A carbocation attacks S. S formal charge (+,0,-) Br formal charge (+,0,-) Ca formal charge (+,0,-) Phrase (A-G) Воx 1: — 0. Box 2: Products: 0arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY