
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
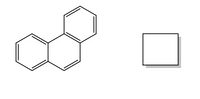
How many additional resonance forms can be drawn for phenanthrene shown below?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The curved-arrow convention depicts the flow of electrons, including bond-forming and breaking events. Draw the outcome of the following reaction based on the provided curved arrow. Be vigilant regarding formal charges. :ci: B. Ci: Draw three additional resonance structures of acetamide (below) and use curved arrow notation to show how the resonance structures are formed. Label the resonance contributors alphabetically (ex. A, B, C, D) and rank them from most to least significant contributor. •oº• Ⅱ. H3C 0°• H N Harrow_forward4) How many electrons are delocalized over how many p-orbitals for the following compound? Which nitrogen atom is more basic? Please explain with help of resonance structures. O N Narrow_forwardProvide the bond line structure of a compound that is able to undergo Allylic lone pair and allylic carbocation resonance. Provide all resonance structures and appropriate arrows. Indicate which resonance state is most stable and why.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided resonance structures, draw the curved electron-pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps.arrow_forward4. Explain why the following molecule is considered aromatic. (it may help to draw a resonance form) O:arrow_forwardDraw three major resonance contributors for the molecule shown in the box. Partiallycompleted skeletal structures are provided as a time-saver; simply add double bonds and formalcharges where appropriate. Do not generate any additional charges.arrow_forward
- Select all that is true for cycloheptatriene and the ions it can form. The 6 pi electrons in cycloheptatriene anion can be delocalized over the ring, which decreases the electronic energy of the molecule. Delocalization of the pi electrons in cycloheptatriene anion increases the electronic energy of the molecule. The cycloheptatriene contains 6 pi electrons, which makes it an aromatic compound. The pi electrons in cycloheptatriene cannot be delocalized over the ring. The pi electrons in cycloheptatriene cation can be delocalized over the ring. The 6 pi electrons in cycloheptatriene cation can be delocalized over the ring, which decreases the electronic energy of the molecule. The cycloheptatriene anion contains 6 pi electrons, which makes it an aromatic compound.arrow_forwardQuestion 2. Draw the molecular orbital diagrams for thioazepine. Indicate if this compound is aromatic, non-aromatic or anti-aromatic using this energy diagram. Justify your answer. N Sarrow_forwardA cationic intermediate for the electrophilic addition of chlorine to the para position of phenol is shown. Draw the resonance structure that is the major contributor. Include all nonbonding electrons. I + -Ö :CI: Select Draw / ||| ||| Templates C More H HOCI Erase Q2Qarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY