
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What carbons are electron deficient and the electron rich? Justify with all the correct resonance structures.
I have figured out 4 resonance structures and know that there are 6 i need to prove.
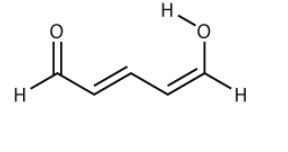
Transcribed Image Text:Н
Н
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1
VIEW Step by stepSolved in 1 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- First, add curved arrow(s) to show the resonance using the following pattern: an allylic lone pair. Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to increase or decrease the charge on an atom, and use the single bond tool to add/remove double bonds. What am I missing here? I've tried putting charges in various spots and still can't get it.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. H HH Select to Add Arrows O..I H3O+ heat HH Select to Add Arrows H3O+ H heat I.O. Please select a drawing or reagent from the question areaarrow_forwardMy book says this compound is not aromatic because it possesses an allene group , which is C=C=C. I dont see where the allene is, can someone show me? I'm confused about how the radical is counted as a C?arrow_forward
- O REPRESENTATIONS OF ORGANIC MOLECULES Ranking resonance structures Rank the resonance structures in each row of the table from major to minor. For example, in the first row, select (major) for the major resonance contributor. If two or more structures in the same row contribute equally, rank them equally by selecting the same number. H. H H. H. H. H. H. H. (Rank) (Rank) (Rank) H. H. H. H. H. H. H. (Rank) (Rank) :o: Rank) (Rank) 74°F DELL F5 F6 F7 F8 F9 F10 F11 PrtScr F12 24arrow_forward2. Draw (in the box) fwo resonance sfrucfures for fhe below anion. — ས CH3arrow_forwardouro- 6)- Draw the structures for the least and most polar molecules (i.e.,isomers) of Bromo-chloro-fl iodoethene (C2BrCIFI). Briefly explain your choice. Structure of least polar: Structure of most polar:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY