
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please help me. reallly appreciated it ?
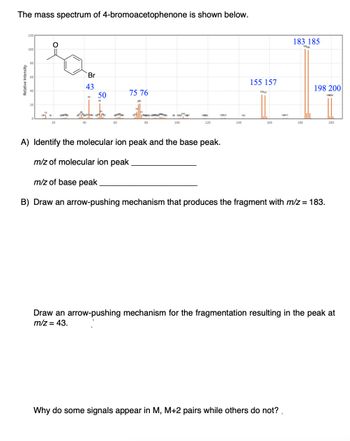
Transcribed Image Text:The mass spectrum of 4-bromoacetophenone is shown below.
Relative Intensity
อ
120
O
Po
Br
43
100
80
60
40
20
15
134 18 29074
20
43
40
50
50
474458 183
60
75 76
786
80
24
98 10021067
100
1139
120
143
140
155 157
1557
160
16371
183 185
18385
Why do some signals appear in M, M+2 pairs while others do not?
180
198 200
A) Identify the molecular ion peak and the base peak.
m/z of molecular ion peak
m/z of base peak
B) Draw an arrow-pushing mechanism that produces the fragment with m/z = 183.
19:00
200
Draw an arrow-pushing mechanism for the fragmentation resulting in the peak at
m/z = 43.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In one experiment, 0.150 L of SnCl4 (d = 2.226 g/mL) was treated with 0.323 L of triethylaluminum (Al(C2H5)3); d = 0.835 g/mL).What is the theoretical yield in this experiment (mass of tetraethylstannane, Sn(C2H5)4)?arrow_forwardO (±)- (±)- (±)- -OME + (±)- ·}- H H. Il H- F HI H I H → ? LOMe H OMearrow_forwardCan you work our KSP for me? thanks Liquid Amount - How many grams of each of the following substances will dissolve in 4.70×102 mL of cold water? Substance - The solubility is 0.123 g/100 mL at 20 oC. ( Ce(IO3)4 )arrow_forward
- Consider the reaction below and answer the question below the reaction. A student performed a synthesis of ethyl 4-aminobenzoate (C) as shown in the reaction above. The student added 1.20 g of 4-aminobenzoic acid and 12.0 mL of ethanol together in the presence of sulfuric acid. The reaction mixture was heated to reflux for a period of 45 minutes. H₂N OH 4-aminobenzoic acid MM 137.14 g/m ol A 77.7% 56.1 % 128 % 72.2 % + CH3CH₂OH ethanol MM 46.10 g/mol H+ H₂N OCH₂CH3 + H₂O ethyl 4-aminobenzoate MM 165.19 g/mol C B Let's assume the theoretical yield of the reaction is 7.22 g and the student produced 5.61 g of ethyl 4-aminobenzoate. What is the percent yield of the reaction?arrow_forwardFumaric bromination 2.5 mL 10% bromine was added to 0.200g of fumaric acid. Calculate the limiting reagent and % yield of the reaction. NB: use density to find the mass of bromine.arrow_forwardConsider the reaction below and answer the question below the reaction. A student performed a synthesis of ethyl 4-aminobenzoate (C) as shown in the reaction above. The student added 1.20 g of 4-aminobenzoic acid and 12.0 mL of ethanol together in the presence of sulfuric acid. The reaction mixture was heated to reflux for a period of 45 minutes. H₂N OH 4-aminobenzoic acid MM 137.14 g/m ol 2.44 g 1.21 g 3.24 g 1.45 g + CH3CH₂OH ethanol MM 46.10 g/mol B H+ H₂N OCH₂CH3 + H₂O ethyl 4-aminobenzoate MM 165.19 g/mol A с What is the theoretical yield for the reaction?arrow_forward
- 2 NO₂(g) → N₂ O₁ (g) O 2511041 O > 0 O ▲,$, O A.S. Q Search 9999 100 # on D KELIAV G 24 Garrow_forwardA Н Н H3C H H Н B CH₁ Lit cu H Br C A Et₂O + ?? 0°C BrMg. В СО BrMg. HH Н.С 2 с CH₂ BrMg. Darrow_forwardity College-CHM 101 (Section 90) - Spring20 - MARX > Activities and Due Dates > Ch 7 HW O Assignment Score: O Resources Ly Give Up? 50.1% O Hint Check Answer < Question 16 of 28 The combustion of ethane (C,H.) produces carbon dioxide and steam. 2 C,H,(s) +70,(g) → 4 CO,(g) + 6 H, O(g) How many moles of CO, are produced when 5.05 mol of ethane is burned in an excess of oxygen? moles of CO,: mol terms of use contactus help about us carecrs privacy policy MacBook Aarrow_forward
- Name the type of reaction CI CI CI CH;CH,C=CCH, CH C CH,CH,C=CCH, CH,Cl, CH,CH,C-CCH, CI Cl CI If only 1 equivalent, if 2 equivalents or eXcess, here reaction reaction to here O Halogenation O Hydrogenation O Hydrohalogenation O Hydrationarrow_forwardRank from most to least reactive in an EAS reaction H 1. II. O=0 Br ||| A.IV>II>I> ||| B.II> IV> | > | C.II>IV>I> ||| D.I>>> IV IVarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY