
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
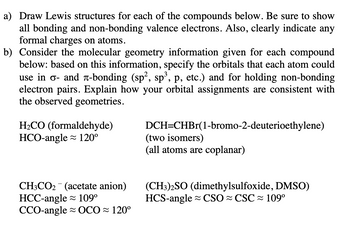
Transcribed Image Text:a) Draw Lewis structures for each of the compounds below. Be sure to show
all bonding and non-bonding valence electrons. Also, clearly indicate any
formal charges on atoms.
b) Consider the molecular geometry information given for each compound
below: based on this information, specify the orbitals that each atom could
use in σ- and à-bonding (sp², sp³, p, etc.) and for holding non-bonding
electron pairs. Explain how your orbital assignments are consistent with
the observed geometries.
H₂CO (formaldehyde)
HCO-angle 120⁰
CH3CO₂ (acetate anion)
HCC-angle ≈ 109⁰
CCO-angle OCO≈ 120°
DCH=CHBr(1-bromo-2-deuterioethylene)
(two isomers)
(all atoms are coplanar)
(CH3)2SO (dimethylsulfoxide, DMSO)
HCS-angle CSO CSC≈ 109⁰
≈
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the number of valence electrons Molecule # 5 SiF?- Draw the Lewis Structure below Draw the three-dimensional structure of the molecule using the "wedge and dash" notation. The molecular geometry is: This molecule is polar nonpolar (Circle your choice.) Our logic for this choice is: The formal charge on Si is The formal charge on F is The hybridization of Si is Estimate F-Si-F bond angle Molecule # 6 CC2F2 Draw the Lewis Structure below Calculate the number of valence electrons Draw the three-dimensional structure of the molecule using the "wedge and dash" notation. The molecular geometry is: This molecule is polar nonpolar (Circle your choice.) Our logic for this choice is: The formal charge on C is The formal charge on Cl/F is The hybridization of C is Estimate F-C-Cl bond anglearrow_forwardConsider the structure Y-X-Y If the symbol X represents a central atom, Y represents outer atoms, and Z represents lone pairs on the central atom, the structure could be abbreviated as XY₂Z₂. 2 Classify each molecule according to its shape. Linear XY₂Z2 Bent (≈ 120°) XY3Z2 Bent (≈ 109°) XY₂Z3 Trigonal pyramidal XY₂Z T-shaped Answer Bank XY3Z See-saw XY4Z2 XY-Z Square planar Square pyramidal XY Zarrow_forward4arrow_forward
- In the molecule shown below, predict the type of hybrid orbitals used by each atom? Question options: (σ-bond), C atom = Sp, (π-bond), C atom = 2p (σ-bond), C atom = Sp2, (π-bond), C atom = Sp2 (σ-bond), C atom = Sp2, (π-bond), C atom = 2p (σ-bond), C atom = Sp3, (π-bond), C atom = Sp2arrow_forwardFor the species below draw additional resonance structures (where all atoms have access to an octect of electrons) for species A, B, C, and D. Determine the bond order of the bond from the underlined C to underlined O for the lowest energy structure(s). You may need to evaluate the formal charge of the additional species you draw. These are species in an aprotic solvent, so only electrons may be moved, not hydrogen atoms. For each lettered structure below, input the bond order as an integer (1,2,etc) or improper fraction (4/3, 5/4, etc). A Structure Bond Order A HO: (f) H B B С Which structure A-D has the longest underlined C-O bond? :N=C=0: D Darrow_forwardcan anyone explain step by step how to predict the molecular geometry for each ion?arrow_forward
- Please helparrow_forward11) Molecular Geometry/ Electron-Group Geometry Total Valence Formula Rough Lewis Structure Electrons Molecular Geometry: SbCl6 Electron-Group Geo.: (Include formal charges other than 0.) More soluble in Bond Polarity (AEN) Molecule Polarity hexane or water? 12) Molecular Geometry/ Electron-Group Geometry Total Valence Formula Rough Lewis Structure Electrons Molecular Geometry: TeF4 Electron-Group Geo.: (Include formal charges other than 0.) More soluble in Bond Polarity (AEN) Molecule Polarity hexane or water? 10arrow_forwardI would like to you help me with my homework because I struggled with step 6. Can you help me with step 6, please?arrow_forward
- ● X E X X View 1 View 2 Which one of your two Views is easier to understand? Explain why? How many different bond angles are there in this geometry. Hint, label each X with a number....e.g. X₁ through X5. A bond angle is then represented by any combination of XAX atoms.arrow_forwardDetermine if the structural formula below is an acceptable Lewis structures for organic compounds. Point out the problems in cases where structure is invalid. :0: CH3 CH-C-Ö-CH–CH3 A. This is not the correct Lewis structure. B. No, All atoms do not have a zero formal charge, therefore this is not the correct Lewis structure. C. All atoms have a zero formal charge, therefore this is the correct Lewis structure. D. Oxygen has a formal charge of 1+.arrow_forwardplease don't provide hand writtin solution....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY