Concept explainers
Question
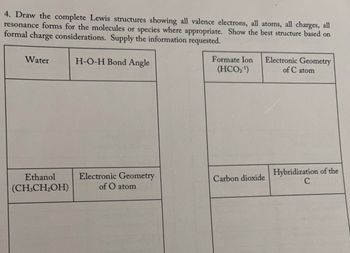
Transcribed Image Text:### Lewis Structures and Molecular Geometry
#### Task
Draw the complete Lewis structures showing:
- All valence electrons
- All atoms
- All charges
- All resonance forms for molecules or species where appropriate
Show the best structure based on formal charge considerations. Provide the information requested.
---
#### Molecules and Information Required
- **Water (H₂O)**
- **H-O-H Bond Angle:**
- Provide the bond angle in degrees.
- **Formate Ion (HCO₂⁻)**
- **Electronic Geometry of C atom:**
- Describe the geometry around the carbon atom.
- **Ethanol (CH₃CH₂OH)**
- **Electronic Geometry of O atom:**
- Describe the geometry around the oxygen atom.
- **Carbon Dioxide (CO₂)**
- **Hybridization of the C atom:**
- Specify the hybridization state of the carbon atom.
---
Note: Ensure all Lewis structures include resonance forms where applicable and minimize formal charges to identify the most stable structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.