
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
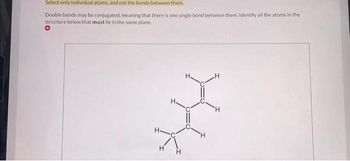
Transcribed Image Text:Select only individual atoms, and not the bonds between them.
Double bonds may be conjugated, meaning that there is one single bond between them. Identify all the atoms in the
structure below that must lie in the same plane.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution with explanation needed..don't give Handwritten answerarrow_forwardDraw the orbital overlap diagram of CH3COOH. Determine which atoms are on the same plane, and which aren't. Explain how you know.arrow_forwardDraw an orbital diagram of the following molecules. Indicate the hybridization of each atom and indicate the orbitals used for each sigma and pi bond. Assign formal charged to each atom.arrow_forward
- Draw the simplest set of curved arrows that shows how the structure on the left could be turned into the structure on the right. Show all lone pairs. If you need to expand part of the structure to show some lone pairs, expand it by drawing in all atoms and bond lines. Br Br toarrow_forwardDescribe the AXE labeling system for VSEPR in detail. What does each letter stand for? For each of the electron and molecular geometries, assign the AXE notation.arrow_forwardWhich of the following properties would you predict that the molecule C7H16 would have based on its structure shown here?arrow_forward
- Predict the splitting pattern of Ha in the structure. На CH-CH3 H3C CH2CH-CH3 H, will be split into a sextet. O None of the other choices. O H, will be split into an octet. O H, will be split into a septet. O H, will be split into a pentet.arrow_forwardConsider three molecules: heptane, trans-3-heptene, and cis-3-heptene. Compare the effect a cis bond has on the overall shape of a 3-heptene molecule to the effect a trans bond has.arrow_forwardpe does this molecule have in idal does this molecule have inarrow_forward
- D a Given that the spatial requirement of a lone pair is greater than that of a bond pair, explain why XeF2 has a linear molecular structure and not a bent one. The four lone pairs of XeF2 occupy the equatorial positions. The angles between lone pairs are 60°, so there is less lone pair/lone pair repulsion with this arrangement. O The two lone pairs of XeF2 occupy the equatorial positions. The angle between lone pairs is 180°, so there is less lone pair/lone pair repulsion with this arrangement. O The two lone pairs of XeF2 occupy the equatorial positions. The angle between lone pairs is 60°, so there is less lone pair/lone pair repulsion with this arrangement. O The three lone pairs of XeF2 occupy the equatorial positions. The angles between lone pairs are 120°, so there is less lone pair/lone pair repulsion with this arrangement. Submitarrow_forwardClick on all of the atoms that make up the largest coplanar unit in the molecule below. H H yht H Harrow_forwardDraw the bond line structure of CH3CH₂COCH(CH3)2 in here. Copy and paste the SMILES code below.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY