
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
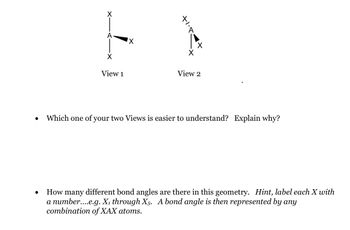
Transcribed Image Text:●
X
E
X
X
View 1
View 2
Which one of your two Views is easier to understand? Explain why?
How many different bond angles are there in this geometry. Hint, label each X with
a number....e.g. X₁ through X5. A bond angle is then represented by any
combination of XAX atoms.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Redraw the molecules in your notebook and circle the more polar compound in each pair of molecules, Hint: You may want to use the elutropic series found in your textbook on p. 182. 1. 6b ба 1b 1a CH, CH, CH, CH, 7b 7a 2b 2a CH, CH3 CH3 OH 8b 8a 3b За CH, HO. H,C. N. CH, 9b 9a 4b 4a CI NH2 HO. H H 10b 10a 5b 5a H N. CH3 CH,. CH3 CH, HO. OH I-Z .. Iarrow_forwardVSEPR HOMEWORK Molecule Lewis Structure # e groups #lone-pair e Hybridization type VSEPR Formula (i.e Molecular Type) Geometric arrangement Molecular Shape Bond Angle Is the molecule Polar? Justify 3d Drawing CIO₂arrow_forwardUsing the table from image 1 D-H can you please fill in the information for the second imagearrow_forward
- I would like to you help me with my homework because I struggled with step 6. Can you help me with step 6, please?arrow_forward1. Although both molecules have 4 F atoms each, SeF4 has _______________ electron group geometry, _________________ molecular geometry and is _____________________, whereas XeF4 has ____________________ electron group geometry, _____________________ molecular geometry and is ______________________. CHOICES: nonpolar trigonal bipyramidal polar seesaw square planar octahedral 2. Match the formula with the approximate bond angle around its central atom 109.5o = ? 120o = ? 90o = ? CHOICES: ClF3 NO2- NH3 3. How many of the following have five (5) electron groups around the central atom? BrF5, PCl5, ClF3, XeF2arrow_forwardI would like to you help me with my homework because I struggled with step 6. Can you help me with step 6, please?arrow_forward
- Identify the molecular and electron geometries around each carbon (left and right). S: H H:C : C:H .. H Left Carbon Right Carbon Mol. Geo.: Mol. Geo.: Electron Geo. Electron Geo: 5.] Use electronegativity values to identify whether the following molecules are polar or nonpolar (explain why?). Indicate the polarity of each bond (towards, or from the central atom) using arrows. The molecular geometry is given in parentheses. (2 points) CH2B12 (tetrahedral geometry with C in the center) Br becavse H- Br Home Seos (trigonal planar with Se in the center) 11 4.arrow_forwardPlease help answer this!arrow_forwardSection 3: Worksheet (continued) 10. CO, Total number of valence electrons: Lewis structure: 3D sketch: Molecular shape / bond angle Polar or nonpolar?arrow_forward
- Options: greater than, less than, same as, nitrogen atom, chlorine atom, less space than, more space than, and the same space as.arrow_forwardDetermine whether each of the molecules below is polar or nonpolar. Linear CO₂ Choose... Linear N₂ Choose... Tetrahedral CH4 Choose... Bent H₂O Choose...arrow_forwardgeNSUIortheastern St OWLv2 LOnline teaching. Chemistry Questions eA. vLAShort Paner: Alcohol/Foo. Centent O X Screenshot 2020-11-15 at 11.03.... O O Q Q ĮKererencesĮ a. Predict the molecular structure and bond angles for SeCle. Approximate bond angles are sufficient. Molecular Structure = Bond Angles b. Predict the molecular structure and bond angles for IC15. Approximate bond angles are sufficient. Molecular Structure = Bond Angles linear bent Try A 3 item attempts remaining ubmit Answer trigonal planar T-shaped trigonal pyramidal tetrahedral square pyramidal square planar octahedral 11:04arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY