
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
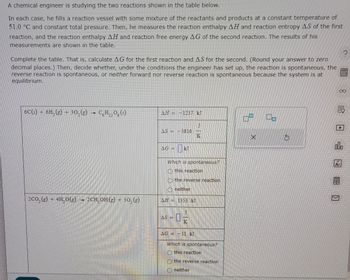
Transcribed Image Text:A chemical engineer is studying the two reactions shown in the table below.
In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of
51.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first
reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his
measurements are shown in the table.
Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero
decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the
reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at
equilibrium.
6C(s) + 6H2(g) + 30, (g)
C6H12O6 (5)
AH=1237. kJ
AS = -3816.
K
2CO2(g) 4H.O(g) → 2CH, OH (g) + 302(g)
AG=
kJ
Which is spontaneous?
this reaction
the reverse reaction
neither
AH = 1353. kJ
AS=
AG = -11 kJ
Which is spontaneous?
(this reaction
the reverse reaction
neither
Ar
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- = ● ENTROPY AND FREE ENERGY Calculating dG from dH and dS A chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 52.0 °c and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy as of the first reaction, and the reaction enthalpy Aн and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and As for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. 2NO(g) + Cl₂(g) → 2NOCI(g) N₂H₂(g) + H₂(g) → 2NH₂(g) ΔΗ - - 76, kJ AS-234. AG = 24 Which is spontaneous? O this reaction O…arrow_forwardA chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 72.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. C₂H₂(g) + 50₂(g) → 3CO₂(g) + 4H₂O(1) 2CO₂ (g) + 4H₂O(g) → 2CH, OH (g) + 30₂ (g) ΔΗ = -1220. ΚΙ J K AS-6432. AG - KJ Which is spontaneous? O this reaction O the reverse reaction…arrow_forwardA chem V ngineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 90.0 °C and constant total pressure. Then. he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. AH = 2220, kJ J AS = 6113. K AG = kJ 3co,() + 4H,0(1) → C,H,(g) + 50,(g) Which is spontaneous? O this reaction O the reverse reaction O neither AH = -951. kJ J AS = AG = – 14. kJ 2A1(s)…arrow_forward
- A chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 144.0°C and constant total pressure. Then, he measures the reaction enthalpy ΔH and reaction entropy ΔS of the first reaction, and the reaction enthalpy ΔH and reaction free energy ΔG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate ΔG for the first reaction and ΔS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. →+C3H8g5O2g+3CO2g4H2Ol =ΔH−2220.kJ =ΔS−5322.JK =ΔGkJ Which is spontaneous? this reaction the…arrow_forwardIn each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 72.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. P₂010 (s) + 6H₂0 (1) 4H₂ PO(s) 4 2NH₂ (g) → N₂H₂(g) + H₂ (g) 2 4 2 ΔΗ = -439. kJ AS-1179. AG = AH = k AS = kJ Which is spontaneous? this reaction the reverse reaction neither 188. kJ OK- K J K AG 16. kJ Which is spontaneous? this reaction the reverse…arrow_forwardA chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 144.0°C and constant total pressure. Then, he measures the reaction enthalpy ΔH and reaction entropy ∆S of the first reaction, and the reaction enthalpy ΔH and reaction free energy ΔG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate ΔG for the first reaction and ∆S for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium.arrow_forward
- A chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 50.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. alo Ar ΔΗ - 439. kJ J AS = - 1359. K ? P,01, (s) + 6H,0(1) . | kJ AG = 4H,PO, (s) Which is spontaneous? this reaction the reverse reaction neitherarrow_forward0/5 Nerbs A chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 85.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. do AH = -2220. kJ J AS = -6189 K C,H, (g) + 50, (g) 3Co, (g) + 4H,0(1) AG = KJ Which is spontaneous? O this reaction O the reverse reaction O neither AH = -50. kJ 0- AS =…arrow_forwardA chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 133.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. 2CH₂OH(g) + 30₂(g) → 2CO₂(g) + 4H₂O(g) 4H₂PO4(s) P4O10 (s) + 6H₂0 (1) AH-1353. kJ AS = -3331. AG = Which is spontaneous? Othis reaction O the reverse reaction Oneither ΔΗ = 439. kJ -…arrow_forward
- A chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 24.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. AH = 188. kJ AS = X Ś ? N₂H₂(g) + H₂(g) → 2NH₂(g) AG = 4 2 P₂(g) + 6Cl₂ (g) 4PC1₂ (g) 688. kJ Which is spontaneous? this reaction the reverse reaction neither AH = - 1207. kJ J AS =…arrow_forwardA chemical engineer is studying the two reactions shown in the table below.. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 124.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. CC14 (8)→ C(s) + 2Cl₂ (g) TICI (g) + 2H₂O(g) → TiO₂ (s) + 4HCl(g) ΔΗ = 107. kJ AS = 310. AG= kJ Which is spontaneous? O this reaction. O the reverse reaction Oneither ΔΗ = -70. KJ…arrow_forwardExplain pleaseeeeeeeeeeearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY