
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
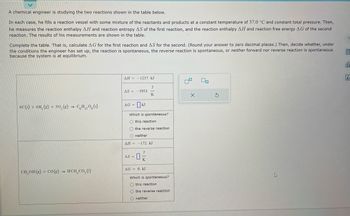
Transcribed Image Text:A chemical engineer is studying the two reactions shown in the table below.
In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 37.0 °C and constant total pressure. Then,
he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second
reaction. The results of his measurements are shown in the table.
Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under
the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous
because the system is at equilibrium.
6C(s) + 6H₂(g) + 30₂(g) → CH₁₂O6 (s)
CH₂OH(g) + CO(g) → HCH₂CO₂ (1)
ΔΗ = -1237. kJ
J
K
AS-3953.
AG = KJ
Which is spontaneous?
O this reaction.
O the reverse reaction
O neither
ΔΗ = − 172. kJ
J
-0%
K
AS =
AG = 0. kJ
Which is spontaneous?
Othis reaction
O the reverse reaction
O neither
4
X
8
00
S
00
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the standard Gibbs free energy change, rG, for the reactions of liquid methanol, of CO(g), and ofethyne, C2H2(g), with oxygen gas to form gaseous carbondioxide and (if hydrogen is present) liquid water at298 K. Use your calculations to decide which of thesesubstances are kinetically stable and which are thermodynamically stable: CH3OH(), CO(g), C2H9(g), CO2(g),H2O().arrow_forwardPredict whether each reaction is reactant-favored or product-favored at 298 K and 1 bar, and calculate the minimum work that would have to be done to force it to occur, or the maximum work that could be done by the reaction. (a) 2 CO2(g) 2 CO(g) + O2(g) (b) 4 Fe(s) + 3 O2(g) 2 Fe2O3(s)arrow_forwardWhat is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the explosive decomposition of TNT? Use your knowledge of TNT and the chemical equation, particularly the phases, to answer this question. (Thermodynamic data for TNT are not in Appendix G.) 2C7H5N3O6(s) 3N2(g) + 5H2O() + 7C(s) + 7CO(g)arrow_forward
- Calculate the standard Gibbs free-energy change when SO3 forms from SO2 and O2 at 298 K. Why is sulfur trioxide an important substance to study? (Hint: What happens when it combines with water?)arrow_forwardAnother step in the metabolism of glucose, which occurs after the formation of glucose6-phosphate, is the conversion of fructose6-phosphate to fructose1,6-bisphosphate(bis meanstwo): Fructose6-phosphate(aq) + H2PO4(aq) fructose l,6-bisphosphate(aq) + H2O() + H+(aq) (a) This reaction has a Gibbs free energy change of +16.7 kJ/mol of fructose6-phosphate. Is it endergonic or exergonic? (b) Write the equation for the formation of 1 mol ADP fromATR for which rG = 30.5 kJ/mol. (c) Couple these two reactions to get an exergonic process;write its overall chemical equation, and calculate theGibbs free energy change.arrow_forwardWhat is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the synthesis of ammonia? 3H2(g) + N2(g) 2NH3(g)arrow_forward
- Chemists and engineers who design nuclear power plants have to worry about high-temperature reactions because it is possible for water to decompose. (a) Under what conditions does this reaction occur spontaneously? 2H2O(g) 2H2(g) + O2(g) (b) Under conditions where the decomposition of water is spontaneous, do nuclear engineers have to worry about an oxygen/hydrogen explosion? Justify your answer.arrow_forwardWhat are the two ways that a final chemical state of a system can be more probable than its initial state?arrow_forwardFor the reaction BaCO3(s) BaO(s) + CO2(g), rG = +219.7 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of fG for BaCO3(s).arrow_forward
- Without looking up their standard entropies in reference tables, identify which of the following lists the materials in order of increasing entropy. (a) H2O() NaCl(s) NH3(g) (b) H2O() NH3(g) NaCl(s) (c) NaCl(s) H2O() NH3(g) (d) NH3(g) H2O() NaCl(s)arrow_forwardThe formation of aluminum oxide from its elements is highly exothermic. If 2.70 g Al metal is burned in pure O2 to give A12O3, calculate how much thermal energy is evolved in the process (at constant pressure).arrow_forwardExplain why absolute entropies can be measured.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning