
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
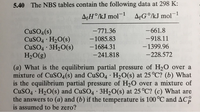
Transcribed Image Text:5.40 The NBS tables contain the following data at 298 K:
A¢H°/kJ mol¬1
AfG°/kJ mol¯
1
-771.36
-661.8
CUSO4(s)
CUSO4 · H2O(s)
CuSO4 · 3H2O(s)
H2O(g)
-1085.83
-918.11
-1684.31
-1399.96
-241.818
-228.572
(a) What is the equilibrium partial pressure of H20 over a
mixture of CuS04(s) and CUSO4 · H2O(s) at 25 °C? (b) What
is the equilibrium partial pressure of H20 over a mixture of
CuSO4 · H20(s) and CUSO4 · 3H2O(s) at 25 °C? (c) What are
the answers to (a) and (b) if the temperature is 100 °C and AC:
is assumed to be zero?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- SO₂ (5.00 g) and CO₂(5.00 g) were placed in a 750.0 mL container at 50.0 °C. The partial pressure of CO2 in the container was atm. Potentially useless information: PV=nRT KE=1/2 mv² R=0.08206 (L'atm)/(mol K) R 8.314 J/(mol K) 760 mm Hg = 760 torr = 1.00 atm = 101 kPa K="C+273 Urms=(3RT/M)1/2 1.60 0.192 6.78 2.76 4.02arrow_forwardCalorimetery practical 7: Q= Calculate the enthalpy of neutralization for ( in units of kJ/mol H2O formed ) for both HCl and HAc. Use all parts : a, b and c. [Must use the normal equation and equation: (Specific heat capacity of water x mass of water x change in temp of water ) / (mass of metal x change in temp of water )] Part A Mass of metal (g) - 10.254 Temperature of boiling water bath (°C) 100.4 Mass of calorimeter (g) 4.044 Mass of calorimeter and water (g) 20.519 Time-zero from graph (s) 45.0 Initial temperature of water from graph(°C) 22.3 Final temp = 29.2 Equation of extrapolation curve from graph- y = –0.0047291x + 29.2 Part B Molarity of NaOH (M) 1 Molarity of HCl (M) 1.1 Mass of calorimeter (g) 3.403 Volume of HCl (mL) 50.0 Volume of NaOH (mL) 50.0 Time-zero from graph (°C) 35.5 Initial temperature of mixture from graph(°C) 22.2 Final temperature 29.3 Equation of extrapolation curve from graph y = –0.0017787x + 29.3 Mass of calorimeter and mixture (g)…arrow_forwardThe cutoff on the left is the temperature rises and the solution has a specific heatarrow_forward
- Use Hess's law to Calculate the AH of the reaction C₂H₂(g) + H₂(g) → C₂H,(g) from the following data. Identify the "manipulation" for each reaction C₂H₂(g) + 3 O₂(g) → 2 CO₂(g) + 2 H₂O(1) C₂H₂(g) + 3% O₂(g) 2 CO₂(g) + 3 H₂O(1) H₂(g) + O₂(g) → H₂O(1) ΔΗ = -1411 kJ AH = -1560 kJ AH = -285.8 kJarrow_forwardThe molar heat of vaporization of some unknown compound (X) at its normal boiling point 8.4 K is 1.6 kcal/mol. Calculate AS (cal/K) for the vaporization of 1.00 mol of X at 8.4 Kand 1 atm. Answer:arrow_forwardA Al(s) + 12(s) = All3(s) В CH4(g) + 4CI2(g) = C(4(1) + 4HCI(g) C Fe,O3(s) + 3CO(g) = 2Fe(s) + 3CO2(g) Which of these processes is AH < 0 OB and C only OA and B only O A, B and OC onlyarrow_forward
- help with physical chemistryarrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B с D H :0: H | || | H с C -C-H 174 H H H chemical symbol, chemical formula or Lewis structure H H H | | C I 1 I H H H (II | Ag - Ar - :O: -O-H boiling point (Choose one) (Choose one) ✓ (Choose one) (Choose one) ✓arrow_forwardIs the following chemical reaction exothermic or endothermic? 31 (aq) + H3AS04(aq) + 2H* (aq) 13 (aq) + H3ASO3(aq) + H2O() AH°F(kJ/mole) S° (J/mole*K) Substance or ion -55.19 180.7 I (aq) -345.69 212.34 H3ASO4(aq) -11.23 11.09 H* (aq) 69.77 -89.6 13 (aq) H3ASO3(aq) -301.27 199.78 -285.8 69.95 H20 (1) endothermic then exothermic All I know is the reaction is cold to the toucharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY