
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Calculate K (2 part question graded as 1)

Transcribed Image Text:For the reaction
H₂S(g) + 2H₂O(1) → 3H₂ (9) + SO₂ (9)
AH° = 295 kJ and AS° = 295 J/K
The equilibrium constant for this reaction at 345.0 K is
Assume that AH° and AS are independent of temperature.
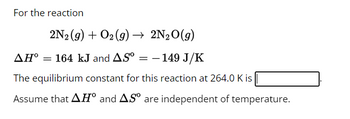
Transcribed Image Text:For the reaction
2N₂ (g) + O2 (g) → 2N₂O(g)
AH° = 164 kJ and AS⁰ = - 149 J/K
The equilibrium constant for this reaction at 264.0 K is
Assume that AH° and AS are independent of temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pls help me asap! make sure to round you final answer to two sig digits. Also don't round any numbers during math only for the final asner plsarrow_forwardal volume of Nach will be needed to 4) If 42.57 mL of 0.10M NaOH is used to back titrate a mixture of an unknown mass of calcium carbonate and 7.67 mL of 3.0M HCl, what was the mass of calcium carbonate in the mixture a. How many milligrams is this?arrow_forwardPhosphate in a solution is titrated with 0.08141 M solution of silver nitrate (AgNO3) as follows:PO 43- + 3Ag+ ---> Ag3PO4How many g of PO43- (94.971 g/mol) are present in the sample if the titration required 30.55 mL of the Standardized Silver Nitrate titrant?Answer to 3 decimal places.arrow_forward
- If a 0.156 g sample of a weak acid requires 25.30 ml of 0.1036 M NaOH to reach an equivalence point, what is the molar mass of the acid? (g/mol) Answer has 3 sig figs.arrow_forwardYou have an aqueous solution of 0.01 M HNO2. What would you expect to happen if you dissolve solid KNO2 in the solution of HNO2? Be sure you canexplain your answer.arrow_forwardG TuT https://app.101edu.co If 24.6 g of KBr (MM = 119.00 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of KBr in the resulting solution? Type here to search F3 K $ Et F5 % n F6 Question 28 of 32 F7 99+ DELL F8 L g & F10arrow_forward
- 2- Suppose a 0.33M aqueous solution of phosphoric acid (H3PO4) is prepared. Calculate the equilibrium molarity of HPO4 properties of phosphoric acid in the ALEKS Data resource. Round your answer to 2 significant digits. Ом x10 You'll find information on thearrow_forwardSubject is Environmental Engineering Please do part darrow_forwarda) Calculate the molarity of Al2(SO4)3 if 0.150 mol Al2(SO4)3 is dissolved in enough water tomake2.70 L of solution. b) Calculate the concentration of each type of ion in the Al2(SO4)3 solution.arrow_forward
- 1.) How Many grams of NaOH (MW=40.0) are in 200 ml of a 0.175 M solution of NaOH?arrow_forwardCalculate the molarity of the H2SO4 solution for each of the three runs.arrow_forwardConte ent attribution Question The end point in a titration of a 42 ml sample of aqueous (CH,COOH) was reached by addition of 14 ml of 0.2 M titrant. The titration reaction is CH3COOH + NaOH → CH3COONA + H2 0 What is the molar concentration of (CH,COOH)? • Round the answer to three significant figures. Provide your answer below: B FEEDBACK SUBMITarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY