
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please answer 49-50
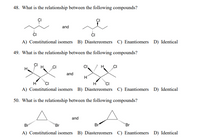
Transcribed Image Text:48. What is the relationship between the following compounds?
and
A) Constitutional isomers B) Diastereomers C) Enantiomers D) Identical
49. What is the relationship between the following compounds?
H,
H.
and
H
H
A) Constitutional isomers B) Diastereomers C) Enantiomers D) Identical
50. What is the relationship between the following compounds?
and
Br
'Br
Br
'Br
A) Constitutional isomers B) Diastereomers C) Enantiomers D) Identical
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please explain the process including the ppm of each peak, thanks!arrow_forwardWhich of the five dyes tested in this experiment has the strongest ability to absorb light? How do you know?arrow_forwardLOD TRANSMITTANCE1% D 4000 O ketone Oester 3000 2000 O carboxylic acid O aldehyde O alcohol O nitrile (i.e., RCN) O None of these other choices is correct. HAVENUMBERI-I 1500 1000 500arrow_forward
- 17) Chemical Formulae: C12H18 8 =0 4H, Doublet Triplet Triplet Doublet elli 18) Chemical Formula: C9H120 IR: Strong broad peak at 3200 cm-¹ PPM 12×2=26-18=-8/2=4 PPM htl=2 Quartet, 2H Singlet, 9H Triplet, 3H 0 0 9x2=18+2=20=12=842arrow_forwardMatch three steroid structures shown below to the following values for Ahexane: Compound A, 275 nm; B, 304 nm, and C, 356 nm. max CH19 C9H19 C3H17 H3C H3C HOarrow_forwardCan you explain this problem? I got it wrong on my exam and don't understand why.arrow_forward
- Isoamyl acetate: Match the peaks to the appropriate number on the structure. A letter may correspond to more than one number. e a b с d 3H 1.7 PPM 1 4 3 3.5 3.0 2.5 2.0 1.5 1.0 0.5 PPM за 1. 1 3 d 2. 2 2 e 3. 3 4 f 4. 4 4 c 5. 5 _1 barrow_forwardI do not know how to find the molecular formulaarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY