
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
- You may draw the structures in either Kekule, condensed, or mixed format initially. In the tables draw them as skeletal structures.
- Attach the sheet of paper with your DEPT spectra at the end of this packet before turning your packet in.
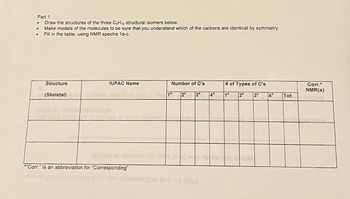
Transcribed Image Text:Part 1.
•
Draw the structures of the three C5H12 structural isomers below.
•
•
Make models of the molecules to be sure that you understand which of the carbons are identical by symmetry.
Fill in the table, using NMR spectra 1a-c.
Structure
IUPAC Name
Number of C's
# of Types of C's
(Skeletal)
Labe 1°
2° 3°
4°
1°
Corr.*
NMR(s)
2° 3°
4°
Tot
*"Corr." Is an abbreviation for "Corresponding"
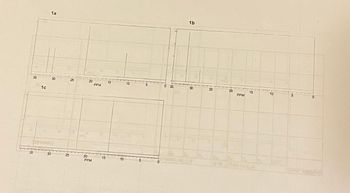
Transcribed Image Text:1a
1b
15
30
25
20
15
10
PPM
0 35
30
35
1c
25
-25
20
20
15
10
PPM
0
35
30
25
20
PPM
15
10
C
(cour waste)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardPredict the major fragment structures and associated mass of each fragment for the following compounds. Submit your answers as a pdf file or ChemDraw file. Oarrow_forwardPlease provide only typed answer solution no handwritten solution needed allowed... Please do it correctly neat and clean Thanksarrow_forward
- 3. In the space below describe how to differentiate between an alcohol and an amine in the IR. 4. In the space below describe how to differentiate between an alcohol and an acid in the IR. 5. In the space below describe how to differentiate between an alkane and an alkene in the IR. 6. In the space below describe how to differentiate between an aldehyde and a ketone in the IR. 7. In the space below describe how to differentiate between an isolated double bond and an aromatic compound such as benzene in the IR. 8. In the space below describe how to differentiate between an ester and a ketone in the IR.arrow_forwardDraw structures for the two fragment ions of highest mass from the following molecule.arrow_forwardGive detailed Solution with explanation needed of all options ...don't give Handwritten answer..arrow_forward
- Which c. ..se structures shows how the pictured molecule will look after it is flipped over horizontally, as illustrated? он flip OH H H. OH он H. ..l OH none of these но Note: Only check the boxes of structures that correspond to a single horizontal flip of the original structure. Ignore structures that have been rotated or flipped multiple times or in other ways.arrow_forwardQuestion 1: Aromaticity a. Draw a fully labelled MO energy diagram for each (match clearly). b. Determine criteria for the following molecules that aligns with the criteria for aromaticity. I will NOT accept anything outside the list done in class – NOTHING YOU HEARD FROM SOMEONE OTHER THAN ME, READ OR SAW ONLINE. List clearly for each molecule the way we did in class. c. Determine if the molecule are aromatic or antiaromatic based on your list. d. Of the two molecules, which would be most reactive in an electrophilic addition? Why?arrow_forwardBetween these 2 compounds, which one will be (R) and which one is (S) configuration? Please draw to explain your answer in detailed..arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY