
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
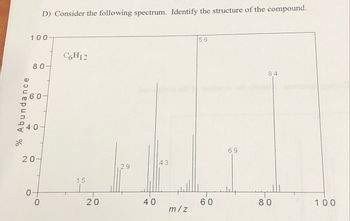
Transcribed Image Text:% Abundance
100-
60
80-
40
20
0
D) Consider the following spectrum. Identify the structure of the compound.
0
C6H12
15
20
29
40
43
m/z
56
60
69
84
80
100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The picture of the question is attachedarrow_forwardA mass spectrum of an unknown compound shows an [M]+ peak with an [M+2]* peak of similar height. Which statement BEST describes the reason for this observation: Bromine atom Two Chorine Atoms Three Chlorine Atoms lodine atom Chlorine atomarrow_forwardIdentify the compound that gives the mass spectrum and infrared spectrum shown here.arrow_forward
- 1. 4 4000 100 50 Identify the following compounds based on the spectra given. ff11 2 20 30 T 0 220 200 180 160 140 2008 CDS-00-094 KAVEMUNERI 40 1500 50 60 120 100 ppm 70 80 500 60 40 20 0arrow_forwardIdentify the molecules and why you think it is the molecule.arrow_forwardWhich of the following compounds (methylene blue, methyl orange, methyl red, or carotene) does the spectrum belong to?arrow_forward
- #9) Using the following spectra and other information provided, identify the compound. Assign pertinent peaks in the spectra. Label the peaks on the spectrum and place the structure of the compound in the box on the right side of the spectrum - Identify correct compound - label proton spectrum - label carbon spectrumarrow_forwardThe simulated APT spectrum of a compound with the molecular formula C6H12 is shown. Draw a structure that is consistent with this data.arrow_forwardwhat is the structure for C5H10O2 given the spectrum belowarrow_forward
- First picture: use the C13NMR spectrum below to determine the structural formula for the compound of molecular formula C5H10O Second picture: picture: use the HNMR spectrum below to determine the structural formula for the compound of molecular formula C6H12O2arrow_forwardmolecular formula: C5H9N 1. Given the molecular formula, label important peaks on the spectrum and explain how you determined the Structural Formula for your assigned compound.arrow_forward#4) Using the following spectra and other information provided, identify the compound. Assign pertinent peaks in the spectra. Label the peaks on the spectrum and place the structure of the compound in the box on the right side of the spectrum - Identify correct compound - label proton spectrum - label carbon spectrumarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning