
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
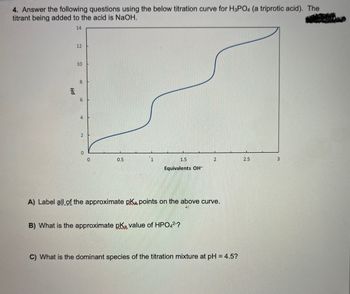
Transcribed Image Text:4. Answer the following questions using the below titration curve for H3PO4 (a triprotic acid). The
titrant being added to the acid is NaOH.
14
Hd
12
10
00
6
4
0
0.5
1.5
Equivalents OH™
B) What is the approximate pKa, value of HPO42-?
2
A) Label all of the approximate pka points on the above curve.
C) What is the dominant species of the titration mixture at pH = 4.5?
2.5
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Ka of HCIO is 3.0x 10-7. When titrating a 50 mL solution of 1 M HCIO with 0.25 M KOH, what will the pH be when you've added 100 mL of the KOH solution? Hint: what point on the titration curve is this?arrow_forwardWhich indicators that would be suitable for each of the following titrations: HNO3 with NaOH thymol blue bromophenol blue methyl orange methyl red chlorophenol blue bromothymol blue cresol red phenolphthaleiarrow_forward12:04 Question 4 of 9 Submit A buffer solution contains dissolved C6H5NH2 and C6H;NH;CI. The initial concentration of C6HŞNH2 is 0.50 M and the pH of the buffer is 4.20. Determine the concentration of C6HŞNH3* in the solution. The value of Kb for C6H;NH2 is 3.8 × 10-1º. 1 2 3 Let x represent the original concentration of C6H,NH,* in the water. Based on the given values, set up the ICE table in order to determine the unknown. C6H5NH2(at H20(1) = OH-(aq) +C6H5NH3*(a Initial (M) Change (M) Equilibrium (M) 5 RESET 0.50 - 4.20 -4.20 6.3 x 10-5 -6.3 x 10-5 1.6 x 10-10 -1.6 x 10-10 3.8 x 10-10 -3.8 x 10-10 x + 4.20 x - 4.20 x + 6.3 x 10-5 х - 6.3 х 10-6 x + 1.6 x 10-10 x - 1.6 x 10-10 x + 3.8 x 10-10 x - 3.8 x 10-10 Tap here or pull up for additional resourcesarrow_forward
- can someone determine the equivalence points of these graphs and explain how you got those values? thank youarrow_forwardIf we have 10 mL of 1.0 M H3PO4 and are titrating it with 1.0 M NAOH, what are the equivalence volumes of NAOH at each of the equivalence points? urite 3 pointsarrow_forwardPart D A 52.0-mL volume of 0.35 M CH3COOH (Ka = 1.8 x 10-5) is titrated with 0.40 M NaOH. Calculate the pH after the addition of 33.0 mL of NaOH. Express your answer numerically. Farrow_forward
- What [NH4Cl] is needed to prepare 1.000 L buffer solution at pH 8.8 using a stock concentration of 0.3500 M NH3? Ka NH4Cl = 5.600 x 10-10 Kb NH3 = 1.800 x 10-5arrow_forwardcan you help with question 4, thank you.arrow_forwardAnswer the following question about a titration between 25.0 mL of a weak acid (Ka= 6.2*10-5) and 0.120 M sodium hydroxide. The pKa of resorcin blue is ~ 5.3and the pKa of chlorophenylmethane~ 8.5. Which indicator would be best to use in the titration?arrow_forward
- You have 0.500 L of an 0.250 M acetate buffer solution (i.e. [HC2H3O2] + [C2H;O2] = 0.250 M) at pH 3.50. How many mL of 1.000 M NaOH must you add in order to change the pH to 5.37? Acetic acid has a pka of 4.74. mL 1 2 3 4 C 7 8 9. +/- х 100 +arrow_forwardDescribe the shape of the titration curve shown, describe why it has this shape.arrow_forwardQuestion is in the image, I got 3.07E-8 mmol protons produced. Need to confirm if this is correct.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY