
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
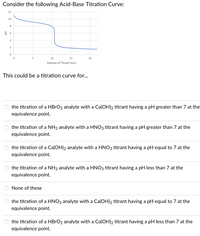
Transcribed Image Text:Consider the following Acid-Base Titration Curve:
12
10
8
공 6
4
10
15
20
Volume of Titrant (mL)
This could be a titration curve for...
the titration of a HBRO2 analyte with a Ca(OH)2 titrant having a pH greater than 7 at the
equivalence point.
the titration of a NH3 analyte with a HNO3 titrant having a pH greater than 7 at the
equivalence point.
the titration of a Ca(OH)2 analyte with a HNO3 titrant having a pH equal to 7 at the
equivalence point.
the titration of a NH3 analyte with a HNO3 titrant having a pH less than 7 at the
equivalence point.
None of these
the titration of a HNO3 analyte with a Ca(OH)2 titrant having a pH equal to 7 at the
equivalence point.
the titration of a HBRO2 analyte with a Ca(OH)2 titrant having a pH less than 7 at the
equivalence point.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 25. mL sample of a weak acid solution (KA= 4.3×10−4) is titrated with 0.20 M NaOH. 20.0 mL of NaOH are needed to reach the equivalence point What is the pH halfway to the equivalence point? 3.37 5.85 4.16 4.74arrow_forwardWhen titrating a 25 mL solution of 0.20M CH3COOH with 0.10M KOH, you realize you forgot to add an indicator initially. You've added 10 mL of KOH to the flask of acid. Ka of CH3COOH = 1.8 x 10^-5 1) Calculate the pH at this point 2) What color would your solution be if you add 40 mL KOH more. And a few drops of clorophenol blue indicator at this point. Why?arrow_forwardWhich one of the following titrations is expected to have a pH < 7 at the equivalence point?arrow_forward
- Consider the titration of 100.0 mL of 0.200 M CH3NH2 by 0.100 M HCI. Calculate the pH after 65.0 mL of HCI added. (K for CH3NH2=4.4 × 10-4) A. 10.96 B. 9.75 C. 7.00 D. 3.68 E. 2.54arrow_forwardA solution of 25.0 mL of 0.095 M hydroxylamonlum lon (weak acid) was titrated with 0.110 M NaOH. The Ka of hydroxylamonium Is 1.1 x 10-6. Calculate the following: What is the volume of NaOH solution at the equivalence point? 21.6 What is the pH after 10 mL of the NaOH is added? 10.85 What is the pH at the equivalence point? 9.85 What is the pH after 25 mL of the NaOH is added? 11.87 X X mLarrow_forwardBewis a titra on curve of a weak acid with NaOH. What region of the titration curve can the volume of NaOH at equivalence point be located? O с B A 2 A B Vol. NaOH (mL) 6 C 8 10arrow_forward
- 12.0 10.0 8.0 pH 6.0 4.0 C 2.0 0.0 D 0 5 10 15 20 25 30 mL of 0.1 M NaOH The graph above shows the titration curves of four monoprotic acids of varying strengths. All of the acids start out at 0.1 M concentration and 25.0 mL volume. Choose all of the correct statements about these titration curves from the cholces below. The titration curve labelled "D" is generated by the strongest acid in the series. In the curves shown above the stronger the acid the less NaOH required for neutralization. The stronger the acid the more important is the pKa of the Indicator used for titration. The Ka of the acid which generates curve "C" is about 103 The weaker the acid the lower the pH of the neutralization point. The acid which generates curve "D" has the highest neutralization-point pH in the series.arrow_forwardIn the titration of a 25.0-mL sample of 0.145 M HCOOH with 0.122 M NaOH, where K, HCOOH = 1.8x10-4 %3D the volume of NAOH added at one-half the equivalence point is about 27.4 mL O the volume of NaOH added at one-half the equivalence point is about 7.45 mL O the volume of NaOH added at one-half the equivalence point is about 12.6 mL O the volume of NaOH added at one-half the equivalence point is about 14.9 mLarrow_forward12.0 10.0 8.0 pH 6.0 4.0 D 2.0 0.0 O 5 10 15 20 25 30 mL of 0.1 M NAOH The graph above shows the titration curves of four monoprotic acids of varying strengths. All of the acids start out at 0.1 M concentration and 25.0 mL volume. Choose all of the correct statements about these titration curves from the choices below. O The titration curve labelled "D" is generated by the strongest acid in the series. O The pk, of the conjugate base of the acid which generates curve "B" is about 6. O The Ka of the acid which generates curve "A" is about 10-5. O Titrating the acid in curve "A" requires the most acidic indicator in the series. O The Ka of the acid which generates curve "B" is about 10-6, The stronger the acid the higher its pH value at the beginning of the titration.arrow_forward
- 23.Determine the pH ranges of the equivalence points in the titration curve below.arrow_forwardCalculating the pH of a weak base titrated with a strong acid 0/5 Izabella An analytical chemist is titrating 165.0 mL of a 0.7400M solution of methylamine (CH3NH2) with a 0.4100M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 318.6 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO 3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ ? 18 Ararrow_forwardClassify each statement as true or false regarding a strong acid/strong base titration. Statement True or false? The steepest part of the titration curve occurs around the equivalence point. The pH at the equivalence point is -7. >arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY