
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
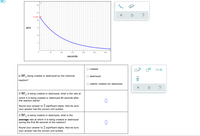
Transcribed Image Text:30-
25
22.285
20
atm
15.
10-
50
100
150
200
250
300
seconds
created
x10
Is BF, being created or destroyed by the chemical
3
destroyed
reaction?
neither created nor destroyed
is being created or destroyed, what is the rate at
If BF3
which it is being created or destroyed 80 seconds after
the reaction starts?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
If BF, is being created or destroyed, what is the
3
average rate at which it is being created or destroyed
during the first 80 seconds of the reaction?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
Expert Solution
arrow_forward
Step 1
The ideal gas law suggest that The volume is directly proportional to the number of moles of the gas, absolute temperature and inversely proportional to the pressure.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwritten solution .....arrow_forwardHere is a graph of the pressure of ethylene (C₂H₂) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. atm 30- 25- 20.870 15- 10+ 5+ 50 100 150 seconds Is C₂H4 being created or destroyed by the chemical reaction? If C₂H4 is being created or destroyed, what is the rate at which it is being created or destroyed 100 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If C₂H4 is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 100 seconds of the reaction? 200 Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. 250 created destroyed 300 neither created nor destroyed 0 X 00 0.0 Śarrow_forwardHere is a graph of the molarity of thionyl chloride (SOC1₂) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. M 0.003 0.00211 0.002 0.001 0 y 10 15 seconds 20 25 30 X Śarrow_forward
- (1 Try Again Your answer is incorrect. • Row 3: Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. Here is a graph of the molarity of formic acid (HCO₂H) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. 3 0.030 0.025 0.020 0.0187 0.015 0.010- 0.005 0 y 500 1000 1500 seconds 2000 2500 3000 × Śarrow_forwardatm 1.50 1.00 0.50 0 5 10 Is NO₂ being created or destroyed by t reaction? 15 seconds Be Careful If NO2 is being created or destroyed, w which it is being created or destroyed 11 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If NO₂ is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 11 seconds of the reaction? 20 Please make sure to use the correct unit symbol. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. 25 created 30 ОК 1.0 atms 1.3 atms - 1 - 1 x10 010 X Sarrow_forwardA particular chemical reaction carried out at 10°C takes 4 hours. Approximately how long will it take to carry out the reaction at 40°C? Question 13 options: A 2 hours B 1 hours C 15 minutes D 30 minutesarrow_forward
- The rate of a reaction is how quickly the reaction goes to completion. If two reactions have the same amount of reactant, increasing the rate does not increase the amount of product produced, it simply reduces the time that it takes to make the product. Using the internet , research the pressure factor below that affect the rate of a chemical reaction. 1. pressure ( be very detailed add graphs to explain your details it should be 300 words ) Your work should be written in complete sentences, and assume your audience is grade 11 chemistry students who do not understand this unit. Make sure all research is put into your own words!arrow_forward= O Kinetics and Equilibrium Calculating average and instantaneous reaction rate from a graph of... Here is a graph of the pressure of boron trifluoride (BF, in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. Explanation Check atm y 30+ 25.724 20- 15 10- 5 0 50 100 150 seconds Is BF3 being created or destroyed by the chemical reaction? If BF is being created or destroyed, what is the rate at which it is being created or destroyed 110 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. in beine If BF is being created or destroyed, what is the 200 250 created destroyed 300 x neither created nor destroyed 0 x10 010 X 0/5 Ś Izabella ? olo 18 Ar 8. © 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardAMoving to another question will save this res Question 20 A graph that displays a straight line when the reciprocal of the concentration is plotted as a function of time. The graph has a slope of 0.482 and a y- intercept of 0.756. What is the rate constant? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation-examples include 1.23 and 12.3 and 120. and -123) Question 20 of 29 AMoving to another question will save this response PIL esc %23 %24 4 & 7. 8 delete R T. Y TO tab enter D. F K return caps lock C V elt control option command optionarrow_forward
- Here is a graph of the pressure of ozone (03) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. atm 3.00- 2.50- 200- 1.950 1.50- 1.00- 0.50- > 0 500 1000 1500 seconds 2000 2500 3000 3arrow_forwardHere is a graph of the molarity of bromine (Br,) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. y 0.003 0.002 0.00184 M 0.001 500 1000 1500 2000 2500 3000 secondsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY