
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
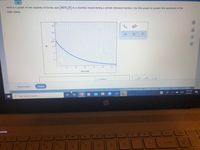
Transcribed Image Text:Here is a graph of the molarity of formic acid (HCO,H)
in a reaction vessel during a certain chemical reaction, Use this graph to answer the questions in the
table below.
T:
0.3+
0.25+
alb
0.2+
Ar
0.187
0.15+
團
0.1+
0.05-
50
100
150
200
250
300
seconds
O created
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use PrivecyI Accessibility
9:03 AM
O Type here to search
2/4/2021
hp
prt sc
celete
home
end
761
144
1508
CT
40
4.
24
41
&
7\
LGO
%23
8.
backsnace
5
Transcribed Image Text:seconds
O created
Is HCO,H being created or destroyed by the chemical
O destroyed
reaction?
O neither created nor destroyed
If HCO,H is being created or destroyed, what is the
rate at which it is being created or destroyed 90
seconds after the reaction starts?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
If HCO,H is being created or destroyed, what is the
average rate at which it is being created or destroyed
during the first 90 seconds of the reaction?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
Explanation
Check
O 2021 McGraw-Hil Education, All Rights Reserved. Terms of Lise
O Type here to search
olo
Expert Solution
arrow_forward
Given
A question about chemical kinetics, which is to be accomplished.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- List the types of chemical reactions, include the generic equations (go back to the video in Module 9 about Types of Chemical Reactions if you need to refresh your memory, here is more practice, if you want to master the topic) . Classify the following chemical reactions in all possible ways (some of the chemical equations are not balanced, do NOT worry about it): C(s) + O₂(g) →→→ CO₂(g) Na(s) + Cl₂(g) →→→ NaCl(g) O H₂O(1) →H₂(g) + O2(8) CaCO3(s) CaO(s) + COz(3) CH4(8) + O₂(8) CO₂(g) + H₂O(g) O C3H8(g) + O2(8) CO2(g) + H₂O(g) O O O O C₂H5OH() + O2(8) Fe(s) + CuCl₂(aq) Cu(s) + AgNO3(aq) BaClz(aq) + Na,SO4(aq) O KBr(aq) +AgNO3(aq) O - O - CO2(8)+ H₂O(g) Cu(s) + FeCl₂(aq) Ag(s) + Cu(NO3)2(aq) →BaSO4(s)+ NaCl(aq) AgBr(s) + KNO3(aa)arrow_forwardCalculate the final molarity of bromide anion in the solution. You can assume the volume of the solution doesn't change when the zinc bromide is dissolved in it. Be sure your answer has the correct number of significant digits.arrow_forwardYou have an unknown solution and you want to determine if it contains ammonia. How will you detect its presence in the laboratory analysis? Please provide two ways to analyze its presence and explain your chosen technique in determining the ammonia.arrow_forward
- Ammonium oxalate dissociates when it dissolves in water. If the concentration of the oxalate ions is 3.2 mol/L, then what is the concentration of the ammonium oxalate solution? Record only your numerical answer with the correct number of significant digits. You do not need to include units as the units appear for you beside the answer box already.arrow_forwardA chemist prepares a solution of vanadium(III) bromide (VBr3) by measuring out 0.10 g of VBr3 into a 100. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br anions in the chemist's solution. Be sure your answer is rounded to 2 significant digits. 0 mol L x10 Xarrow_forwardA chemist prepares a solution of potassium bromide KBr by measuring out 0.17g of KBr into a 300.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br− anions in the chemist's solution. Be sure your answer is rounded to 2 significant digits.arrow_forward
- An analytical chemist weighs out 0.159 g of an unknown monoprotic acid into a 250 mL volumetric flask and dilutes to the mark titrates this solution with 0.0700 M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 25.2 mL of NaOH solution. Calculate the molar mass of the unknown acid. Round your answer to 3 significant digits. molarrow_forwardA student wants to make a 0.600 M aqueous solution of barium sulfate, BaSO4, and has a bottle containing 12.00 g of barium sulfate. What should be the final volume of the solution? Find the numerical answer for this question and make sure to include the following: What is the formula for molarity? What is the molar mass for barium sulfate? When you give your numerical answer, what are the correct significant figures and how do you know that is the correct amount?arrow_forwardComplete and balance each of the following equations for gas-evolution reactions. Part A HNO3(aq)+Na2SO3(aq)→ Express your answer as a chemical equation. Part B HCl(aq)+KHCO3(aq)→ Express your answer as a chemical equation. Part C HI(aq)+KHSO3(aq)→ Express your answer as a chemical equation. Part D (NH4)2SO4(aq)+KOH(aq)→ Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forward
- In the laboratory you dissolve 14.7 g of chromium(III) nitrate in a volumetric flask and add water to a total volume of 250 mL.What is the molarity of the solution? What is the concentration of the chromium(III) cation? What is the concentration of the nitrate anion?arrow_forwardFor each of the reactions, calculate the mass (in grams) of the product formed when 15.67 g of the underlined reactant completely reacts. Assume that there is more than enough of the other reactant. 2K(s)+Cl2(g)−−−−−→2KCl(s) 2K(s)+Br2(l)−−−−−→2KBr(s) 4Cr(s)+3O2(g)−−−−−→2Cr2O3(s) 2Sr(s)−−−−+O2(g)→2SrO(s)arrow_forwardYou need to make an aqueous solution of 0.196 M iron(III) sulfate for an experiment in the lab, using a 250 mL volumetric flask. How much solid iron(III) sulfate should you add?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY