
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
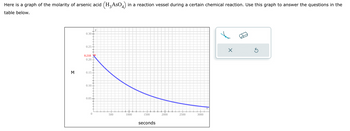
Transcribed Image Text:Here is a graph of the molarity of arsenic acid (H₂AsO4) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the
table below.
M
0.30-
0.25-
0.218
0.20
0.15+
0.10
0.05-
0
500
1000
1500
seconds
2000
2500
3000
X

Transcribed Image Text:If H3 ASO4 is being created or destroyed, what is the
rate at which it is being created or destroyed 1100
seconds after the reaction starts?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
If H3 AsO is being created or destroyed, what is the
average rate at which it is being created or destroyed
during the first 1100 seconds of the reaction?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Volumetric Analysis: Determination of Acetic Acid in Vinegar You measured the vinegar volumetrically in procedure B1, what piece of data was needed to determine the volume needed from the mass of vinegar?arrow_forwardHere is a graph of the molarity of glucose (C6H₁2O6) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. M 0.30- 0.25- 0.195 0.10- 0.05- 0 500 1000 1500 seconds 2000 2500 # 3000 X Œ 3arrow_forwardA reaction is made up in the following way: 16 mL of 4.3 M acetone + 16 mL of 1.1 M HCI + 16 mL of 0.0058 M I2 + 14 mL of water. What was the inital concentration of I2 in the reaction mixture? Express your answer as a decimal number (no exponents). Please include proper (abbreviated) units.arrow_forward
- An aqueous solution at 25 °C has a H₂O concentration of 6.2 × 10¯M. Calculate the OH concentration. Be sure your answer has the correct number of significant digits. -7 1.61 × 10 M x10 X Śarrow_forwardIf 2.0 × 10-4 moles of H2O2H2O2 in 50 mL solution is consumed in 3 minutes and 25 seconds, what is the rate of consumption of H2O2H2O2? Express the rate in mol per liter·second to two significant digits.arrow_forwardA student wants to make a 0.600 M aqueous solution of barium sulfate, BaSO4, and has a bottle containing 12.00 g of barium sulfate. What should be the final volume of the solution? Find the numerical answer for this question and make sure to include the following: What is the formula for molarity? What is the molar mass for barium sulfate? When you give your numerical answer, what are the correct significant figures and how do you know that is the correct amount?arrow_forward
- A chemistry student must write down in her lab notebook the concentration of a solution of sodium thiosulfate. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 5.0 g She put some solid sodium thiosulfate into the graduated cylinder and weighed it. With the sodium thiosulfate added, the cylinder weighed 61.167 g. • She added water to the graduated cylinder and dissolved the sodium thiosulfate completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 10.64 mL. What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits. - 1 g•mL х10arrow_forwardcom/alekscgi/x/Isl.exe/1o_u-IgNslkr/J8P3JH-IBEWe Maps O MEASUREMENT Lindsay Adding or subtracting and multiplying or dividing measurements chemistry student must write down in her lab notebook the concentration of a solution of sodium hydroxide. The concentration of a solution equals the mass what's dissolved divided by the total volume of the solution. re's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 6.050 g dlo - She put some solid sodium hydroxide into the graduated cylinder and weighed it. With the sodium hydroxide added, the cylinder weighed 96.4 g. - She added water to the graduated cylinder and dissolved the sodium hydroxide completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 151.66 mL. Ar at concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits. -1 gmLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY