
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
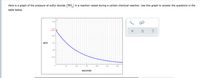
Transcribed Image Text:Here is a graph of the pressure of sulfur dioxide (SO,) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the
table below.
y
3.00
2.50–
2.417
2.00-
atm
1.50-
1.00-
0.50-
50
100
150
200
250
300
seconds

Transcribed Image Text:created
x10
Is SO, being created or destroyed by the chemical
destroyed
reaction?
neither created nor destroyed
If SO, is being created or destroyed, what is the rate at
which it is being created or destroyed 70 seconds after
the reaction starts?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
If SO, is being created or destroyed, what is the
average rate at which it is being created or destroyed
during the first 70 seconds of the reaction?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 12.105 Using the kinetic-molecular theory, explain why an increase in pressure produces more N2O4 in the following system: N2O4(g)2NO2(g)arrow_forwardXenon and fluorine will react to form binary compounds when a mixture of these two gases is heated to 400C in a nickel reaction vessel. A 100.0-mL nickel container is filled with xenon and fluorine, giving partial pressures of 1.24 atm and 10.10 atm, respectively, at a temperature of 25C. The reaction vessel is heated to 400C to cause a reaction to occur and then cooled to a temperature at which F2 is a gas and the xenon fluoride compound produced is a nonvolatile solid. The remaining F2 gas is transferred to another 100.0-mL nickel container, where the pressure of F2 at 25C is 7.62 atm. Assuming all of the xenon has reacted, what is the formula of the product?arrow_forwardAs weather balloons rise from the earths surface, the pressure of the atmosphere becomes less, tending to cause the volume of the balloons to expand. However, the temperatura is much lower in the upper atmosphere than at sea level. Would this temperatura effect tend to make such a balloon expand or contract? Weather balloons do, in fact, expand as they rise. What does this tell you?arrow_forward
- A typical barometric pressure in Redding. California, is about 750 mm Hg. Calculate this pressure in atm and kPa.arrow_forward105 The decomposition of mercury(II) thiocyanate produces an odd brown snake-like mass that is so unusual the process was once used in fireworks displays. There are actually several reactions that take place when the solid Hg(SCN)2 is ignited: 2Hg(SCN)2(s)2HgS(s)+CS2(s)+C3N4(s)CS2(s)+3O2(g)CO2(g)+2SO2(g)2C3N4(s)3(CN)2(g)+N2(g)HgS(s)+O2(g)Hg(l)+SO2(g) A 42.4-g sample of Hg(SCN)2 is placed into a 2.4-L vessel at 21°C. The vessel also contains air at a pressure of 758 torr. The container is sealed and the mixture is ignited, causing the reaction sequence above to occur. Once the reaction is complete, the container is cooled back to the original temperature of 21°C. (a) Without doing numerical calculations, predict whether the final pressure in the vessel will be greater than, less than, or equal to the initial pressure. Explain your answer. (b) Calculate the final pressure and compare your result with your prediction. (Assume that the mole fraction of O2 in air is 0.21.)arrow_forward93 The complete combustion of octane can be used as a model for the burning of gasoline: 2C8H18+25O216CO2+18H2O Assuming that this equation provides a reasonable model of the actual combustion process, what volume of air at 1.0 atm and 25°C must be taken into an engine to burn 1 gallon of gasoline? (The partial pressure of oxygen in air is 0.21 atm and the density of liquid octane is 0.70 g/mL.)arrow_forward
- A cylinder of compressed gas is labeled Composition (mole %): 4.5% H2S, 3.0% CO2, balance N2. The pressure gauge attached to the cylinder reads 46 atm. Calculate the partial pressure of each gas, in atmospheres, in the cylinder.arrow_forwardThe graph below is similar to that of Figure 12.2. If after 100 s have elapsed the partial pressure of N2O4 is increased to 1.0 atm, what will the graph for N2O4 look like beyond 100 s?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
