
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
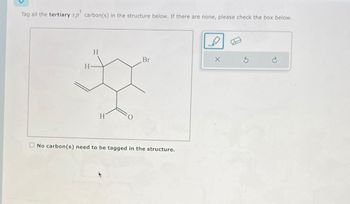
Transcribed Image Text:Tag all the tertiary sp³ carbon(s) in the structure below. If there are none, please check the box below.
H
H
H
Br
No carbon (s) need to be tagged in the structure.
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Tag all the 3° sp carbon(s) in the structure below. If there are n Br OH No carbon(s) need to be tagged in the structure.arrow_forwardUsing the numbering scheme below, which carbons are secondary (2°)? C 2 12 C 3 1 [] 2 3 st 4 5 06 ים 8 6 8arrow_forwardCheck the box under each molecule in the table below that is an isomer of this molecule: OH OH If there are no isomers, check the none of the above box under the table. HO OH OH Onone of the above R да HO OH X 5arrow_forward
- ✓eck the box under each molecule in the table below that is an isomer of this molecule: i HO If there are no isomers, check the none of the above box under the table. HO OH none of the above O 0 OH X Sarrow_forward5arrow_forwardFor the molecule shown select all the substituents that are trans to the Br group. Br Ме он H, tBu I NH2 Ha Et Clarrow_forward
- Correct each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolved in a 0.1 M aqueous solution of HC1. If there are no changes to be made, check the No changes box under the drawing area. CH-C-OH No changes. HO–CH–CH, NH, - × : Garrow_forwardSelect the correct number of primary, secondary, tertiary and quaternary carbons that are within the molecule shown here (You will need to make 4 selections): O 2 Secondary O 5 O 2 Quaternary O 3 Quaternary O 5 O 2 Primary O 3 Secondary Tertiary Tertiary Tertiary Quaternary Primary Primary Secondaryarrow_forward7.arrow_forward
- Check all of the compounds in the list below that are constitutional (structural) isomers of each other. О Н +н Н H Н Н H С. C H # -Н Н H Н Н Н Н Н- Н Н C Н Н Н Н te Н OH Н None of the Above Н H Н Harrow_forwardCan you help me find all of the electron geometry tetrahedral bonds in this Vitimine A structure? The photo attached is one I got wrong. Thank you.arrow_forwardPart 1 of 2 Which of the following molecules is/are constitutional isomer(s) to (CH3) CHCH₂CH₂C(CH₂)₂? Check all that 2 apply. > CH₂ 3 CH₂ 3 I HỌC–CH–CH,-CH,-C−CH, None of the above CH3 (CH₂)₂C(CH₂)₂C(CH₂)₂ 3 X OO olo Ar ▶arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY