
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can you please answer all the following questions

Transcribed Image Text:**Educational Exercise:**
### Chirality and Stereoisomers
1. **Exercise Task:**
**Objective:** Redraw the given molecule ensuring all chirality centers exhibit the S configuration.
**Molecule Provided:**
- The molecule features multiple functional groups, including alcohol (OH) and carboxylic acid (COOH).
- It contains a six-membered carbon ring structure with various substituents like fluorine (F) and a nitrogen-containing moiety.
**Instructions:** Utilize your understanding of stereochemistry to redraw the molecule, ensuring that each chiral center is in the S configuration.
**Diagram Placeholder:** A blank space is provided for redrawing the molecule with the specified stereochemistry.
2. **Further Exploration: Stereoisomers of Dichlorocyclopentane**
**Task:**
a. Draw a stereoisomer of dichlorocyclopentane (C₅H₈Cl₂) that is a meso compound.
b. Draw another isomer of the same compound that lacks a chirality center.
**Understanding Meso Compounds:**
- Meso compounds have multiple stereocenters but are achiral due to an internal plane of symmetry.
**Instructions:**
- For the meso compound: Sketch the dichlorocyclopentane in a way that two chiral centers cancel each other’s optical activity due to symmetry.
- For the isomer without a chirality center: Arrive at a structural arrangement where the stereocenters are eliminated or do not contribute to chirality.
**Diagram Placeholders:** Two spaces provided for the illustrations of the requested isomers.
Use this exercise to deepen your understanding of stereochemistry and the intricacies of chirality and stereoisomers.
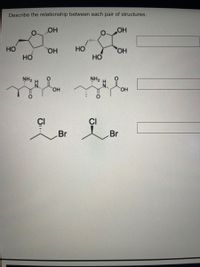
Transcribed Image Text:**Title: Understanding the Relationship Between Chemical Structures**
**Description:** This educational module focuses on identifying and describing the relationship between different pairs of chemical structures. Here, we examine three pairs of compounds, looking at their similarities and differences.
---
**Image Analysis:**
1. **Pair 1:**
- **Left Structure:** A five-membered ring with one oxygen and four carbons. It has hydroxyl (OH) groups attached at various positions.
- **Right Structure:** Similar five-membered ring with identical functional groups but varies in spatial arrangement.
**Explanation:** This pair consists of stereoisomers, where the spatial arrangement of atoms creates different configurations.
2. **Pair 2:**
- **Left Structure:** A molecule with a chain of carbons, featuring an amine group (NH₂) and a carboxylic acid (COOH) group.
- **Right Structure:** Appears nearly identical; however, look for changes in the configuration around specific bonds or atoms.
**Explanation:** This pair could represent structural isomers, differing in the arrangement of atoms but having the same molecular formula.
3. **Pair 3:**
- **Left Structure:** A simple carbon chain with a chlorine (Cl) and a bromine (Br) atom attached.
- **Right Structure:** Similar molecular structure with the positions of Cl and Br interchanged.
**Explanation:** This is a classic example of positional isomers, where halogen substituents switch positions on the carbon chain.
---
**Conclusion:** Each pair illustrates a fundamental concept in chemistry involving the spatial and structural arrangement of atoms within molecules. Understanding these relationships is crucial for recognizing how molecular changes can affect chemical properties and reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of methyl acetate, chloroform, ethanolamine, diethylamine, and tetrahydrofuran. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid methyl acetate chloroform ethanolamine diethylamine tetrahydrofuran density 0.93 g ml 1.5 g ml 1 -1 1.0 g ml 1 0.71 g ml 0.89 g ml. Next, the chemist measures the volume of the unknown liquid as 1005. cm' and the mass of the unknown liquid as 1.49 kg. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. Given the data above, is it possible to Identify the liquid? If it is possible to identify the liquid, do so. 0 gml. yes no methyl acetate chloroform ethanolamine…arrow_forwardhow do i find the freezing point of this graph?arrow_forwardThe study of detecting small quantities of substances in samples would most likely be performed by a chemist in the branch of chemistry known as ___ . organic chemistry biochemistry inorganic chemistry analytical chemistry physical chemistryarrow_forward
- What is green chemistry? Choose the BEST answer. O Chemistry that uses only recycled chemicals. O Chemistry that makes something green in color. O Chemistry that comes from plants or uses plant materials. O Chemistry that uses non-toxic, non-harmful chemicals.arrow_forwardExplain if it is possible to combine different types of mass analyzer.arrow_forwardWhat is calibration and why is it essential in relation to food analysis? Provide examples.arrow_forward
- A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of chloroform, ethanolamine, dimethyl sulfoxide, pentane, and carbon tetrachloride. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid chloroform density 1.5 g cm 3 ethanolamine 1.0 g-cm dimethyl sulfoxide 1.1 g cm pentane 0.63 g-cm carbon tetrachloride 1.6 g-cm Next, the chemist measures the volume of the unknown liquid as 0.688 L and the mass of the unknown liquid as 543. g. Calculate the density of the liquid. Round your answer to 3 significant digits. Given the data above, is it possible to identify the liquid? If it is possible to identify the liquid, do so. gcn cm yes no chloroform ethanolamine dimethyl sulfoxide pentane carbon tetrachloride X 5…arrow_forwardWhat is the density of polyethylene (in g/mL) to two significant figures at 25 ∘C?arrow_forwardYou have a 11.5 mg sample of blood that contains various proteins. Hemoglobin is the only protein in the sample containing Fe. Hemoglobin contains 3.83% by mass of a compound called heme (C₃₄H₃₂FeN₄O₄, MW = 616.49 g/mol). You find that your 11.5 mg blood sample contains 34.9 micrograms of Fe. What is the mass percent of hemoglobin in your protein sample? -don't prematureley roundarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY