
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
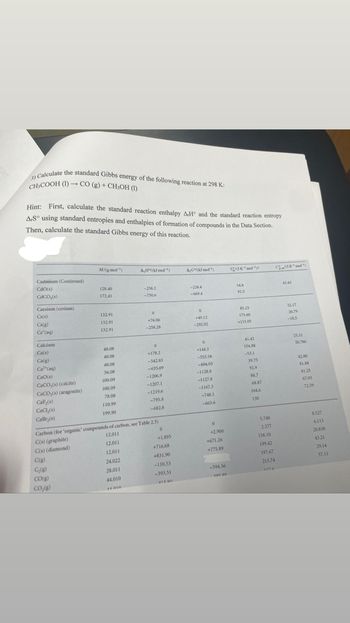
Transcribed Image Text:3) Calculate the standard Gibbs energy of the following reaction at 298 K:
CH₂COOH (1)→ CO (g) + CH3OH (1)
Hint: First, calculate the standard reaction enthalpy AH and the standard reaction entropy
AS using standard entropies and enthalpies of formation of compounds in the Data Section.
Then, calculate the standard Gibbs energy of this reaction.
Cadmium (Continued)
Cdo(s)
CaCO,(s)
Caesium (cesium)
Cs(s)
Cs(g)
Cs' (aq)
Calcium
Ca(s)
Ca(g)
Ca (aq)
CIO(8)
CaCO,(s) (calcite)
CaCO,(s) (aragonite)
CaF₂(s)
CaCl₂(8)
CaBry(s)
M/(g mol-¹)
C₂(g)
CO(g)
CO₂(g)
128.40
172.41
132.91
132.91
132.91
40.08
40.08
40.08
56.08
100.09
100.09
78.08
110,99
199.90
AH/(kl mol-¹)
-258.2
-750.6
0
+76.06
-258.28
0
+178.2
-542.83
-635.09
-1206.9
-1207.1
-1219.6
-795.8
-682.8
0
+1.895
+716.68
+831.90
-110.53
-393.51
413 on
Carbon (for 'organic' compounds of carbon, see Table 2.5)
C(s) (graphite)
12.011
C(s) (diamond)
12.011
C(g)
12.011
24.022
28.011
44,010
14010
AGKJ mol¹)
-228.4
-669 A
0
+49.12
-292.02
0
+1443
-553.58
-604.03
-1128.8
-1127.8
-1167.3
-748,1
-663.6
0
+2.900
+671.26
+775.89
-394.36
105 00
$20K molt
54.8
92.5
85.23
175.60
+133.05
41.42
154.88
-53.1
39.75
92.9
88.7
68.87
104.6
130
5.740
2.377
158.10
199.42
197.67
213.74
1176
CK¹mot')
43.43
32.17
20.79
-10.5
25.31
20.786
42.80
81.88
81.25
67.03
72.59
8.527
6.113
20.838
43.21
29.14
37.11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 37.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. 6C(s) + 6H₂(g) + 30₂(g) → CH₁₂O6 (s) CH₂OH(g) + CO(g) → HCH₂CO₂ (1) ΔΗ = -1237. kJ J K AS-3953. AG = KJ Which is spontaneous? O this reaction. O the reverse reaction O neither ΔΗ = −…arrow_forwardWhat is the standard Gibbs free energy for the transformation of diamond to graphite at 298 KK? Express your answer to three significant figures and include the appropriate units.arrow_forwardDiscuss the relations between: the entropy (particularly of the universe), the standard Gibbs' free energy change, the spontaneity, the equilibrium constant, and the equilibrium position for or associated with a process.arrow_forward
- Calculate the entropy change in surroundings when 1.00 mol of H:0 (1) is formed under standard conditions, A:H = -286 kJmol1. Industrially, methanol is synthesized using the reaction Co(e) + 2 H2(g) =CH:OH(g) Calculate the equilibrium constant at 298 K. AGP CO(g) = -137.2 kJ/mol; AGP H: (g)= 0; AGP CH:OH(g) = -162 kJ/molarrow_forward(16) Solid potassium chlorate decomposes into solid potassium chloride and oxygen according to the following balanced chemical equation: KCIO, (s) → KCI (s) + 3/2 O, (g) Given the enthalpy of reaction is -77.6 kl and the entropy of reaction is 494.6 3/K, the overall Gibbs free energy change for this reaction at 40.0°C is: (A) (B) (C) (D) (E) -252 k -157 kJ -1.55 x 10 kJ -232.5 kJ -97.4 kJarrow_forwardUse the data from this table of thermodynamic properties to calculate the maximum amount of work that can be obtained from the combustion of 1.00 mole of ethane, CH3CH3(g), at 25 °C and standard conditions. moarrow_forward
- Make a non-spontaneous process spontaneous A process that is nonspontaneous can be made spontaneous by coupling it with another process tha is highly spontaneous. The coupling of nonspontaneous reactions with highly spontaneous ones is important in biological system. The oxidization of glucose, for example, is highly spontaneous: C6H12O6(s) + 60₂(g) → 6 CO₂(g) + 6 H₂O(1) Spontaneous reactions such as this ultimately drive the nonspontaneous reactions necessary to sustain life. Can you use Gibbs free energy, enthalpy, and entropy to argue this reaction is highly spontaneous?arrow_forward-What are standard transformed states of Gibbs Free Energy and the Equilibrium Constant and why is this significant in biochemistry? -What is the definition of the Equilibrium Constant?arrow_forwardHalf a mole of a perfect gas expands isothermally and at 298.15 K from a volume of 10 L to avolume of 20 L. (a) What is the change in the entropy of the gas? (b) How much work is doneon the gas? (c) What is the heat of Surroundings ? (d) What is the change in the entropy of the surroundings? (e)What is the change in the entropy of the system plus the surroundings?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY