
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Pls help asap
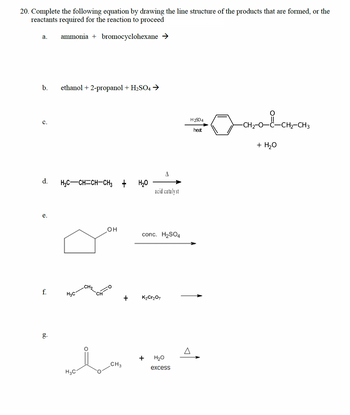
Transcribed Image Text:20. Complete the following equation by drawing the line structure of the products that are formed, or the
reactants required for the reaction to proceed
a.
ammonia + bromocyclohexane →
b.
c.
ethanol + 2-propanol + H2SO4 →
A
d.
H3C-CH=CH-CH3 +
H₂O
acid catalyst
e.
OH
conc. H2SO4
CH₂
f.
H3C
K2Cr2O7
g.
+
H₂O
CH3
excess
H3C
H2SO4
heat
✓ CH₂-O-PL-CH2-CH3
+ H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- From among the given compounds, choose the reactant(s) for each of the reactions described below and write the chemical equation for the reaction. The given compounds may be used more than once. The structure of the reactant(s), major organic product and the specific inorganic reagent/reaction conditions must all be shown. o OH CH₂ -CI C(CH₂)3 CH₂ 12 For OH CH₂ CH₂CH₂ 1. generation of an alkoxide ion with rapid bubbling rate 2. an S1 reaction of a chiral alkyl halide using sodium methoxide in methanol 3. E2 reaction of an alkyl halide to give a disubstituted alkene 4. Williamson ether synthesis to produce a cycloalkyl ether Brarrow_forwardWhat is the product of this reaction? НО HO. H2SO4, Heatarrow_forward3- Write the organic product(s) and reaction mechanism a- b- CO₂H H3O heat CO₂CH3 1. NaOCH3 2. (CH3)2CHI 3. aq. NaOH 4. H₂O*, heatarrow_forward
- Find the product of this reaction 1. CH₂O, to c) 2. CH,COOOH ILM 2. H₂O 1. C₂H,MgBr 2. H₂O 3. H₂SO, to HBr, peroxide (CH3) COK, to 2. O 4. H₂O2 Mg. etherarrow_forwardEthanol (CH3CH2OH) is the alcohol found in beverages. It is oxidized in the body to acetaldehyde by the enzyme alcohol dehydrogenase. Methanol (CH3OH), also known as wood alcohol, is converted to formaldehyde by the same enzyme. Acetaldehyde is toxic, but formaldehyde is far more toxic to humans, which is why the ingestion of relatively small amounts of methanol can cause blindness or death. One treatment for mild methanol poisoning is the administration of ethanol. Why might a doctor choose this treatment? A. Ethanol likely irreversibly binds to alcohol dehydrogenase which prevents the formation of formaldehyde. B. The doctor has given up on the patient and administers ethanol for sedation. C. Ethanol must act as a competitive inhibitor for the alcohol dehydrogenase and therefore slows the formation of formaldehyde. D. The ethanol is likely an uncompetitive inhibitor and binds to a site other than the active site of the enzyme.arrow_forward10. Classify the following alcohols as primary, secondary, or tertiary a. b. LOH d. OH OH OHarrow_forward
- The chemical which neutralizes HCHO, it can be used to neutralize a large spill for safe cleanup, is ___. organic acids paraformaldehyde ammonia EDTAarrow_forwardAcetylsalicylic acid (aspirin) can be synthesized by combining salicylic acid and acetic acid through a condensation reaction. The-OH group from phenol on the salicylic acid condenses with the carboxylic acid group of acetic acid forming acetylsalicylic acid OH OH HO CH₂ Condensation reactionarrow_forwardtype of organic reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning