
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
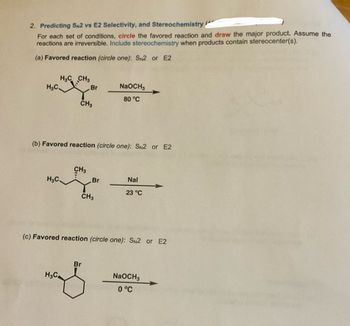
Transcribed Image Text:2. Predicting SN2 vs E2 Selectivity, and Stereochemistry
For each set of conditions, circle the favored reaction and draw the major product. Assume the
reactions are irreversible. Include stereochemistry when products contain stereocenter(s).
(a) Favored reaction (circle one): SN2 or E2
HạC CH,
H.C
Br
NaOCH3
Y
80 °C
CH3
(b) Favored reaction (circle one): SN2 or E2
H3C.
CH3
CH3
Nal
Br
23 °C
(c) Favored reaction (circle one): SN2 or E2
Br
H3C
was
NaOCH3
0°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 1. Draw the product of each of the following [3,3] sigmatropic rearrangements, including its stereochemistry. Ph CH3 (a) H3C. (b) Ph CH3 (c) H3C CO₂CH3 CH3 H (d)arrow_forwardb) DRAW THE product of each of the following reactions? DRAW expected product stereochemistry, where NEEDED TO b) חוו H3C- Pd(OAc)2 Ag2CO3 dppe H OBn 1. B2H6, THF CH3 CH3 2. NaOH, H2O2 B 0arrow_forwardWhen norbornene undergoes hydroboration-oxidation, a mixture of two stereoisomers is produced in a roughly 6:1 ratio. (a) Draw both of these isomeric products. (b) Which product is favored? Hint: You should build a model of norbornene and consider the transition state leading to each product. 1. BH3 THF ? 2. H2O2, NaOH, H,O Norbornenearrow_forward
- Q1: Determine the major product(s) of the following reaction with correct stereochemistry where applicable. H2SO4 (cat.) H) XH OH heat Br 1) MeOH SN1 Q2. Ranke the following in the order of increasing reactivity towards SN1 reaction. CH3 CH3 CH3 H2C CH2 CH3 CH3 H₂C CH2 CH3 CH3 H2C CH2 H3C Br H3C Br H3C CI Br I ။ III IV Least reactive Most reactivearrow_forwardFor the following dehydrohalogenation (E2) reaction, draw the major organic product(s), including stereochemistry CI (Cн Сок СH, HII (Cн, СОнarrow_forwardPlease show the major products with appropriate stereochemistry where needed.arrow_forward
- 1a. Provide the products/reactants of the SN1 reactions below. Be very careful of the stereochemistry if applicable. (Recall that in SN1 reaction, the reactant’s chirality (aka configuration of the halo-carbon) is converted to both enantiomer – so you might need show both structures with dashed and wedge bonds 1b. Draw the mechanism (use curved arrow) of of EITHER c or d abovearrow_forward3. Complete the following reaction scheme. Give all product(s) and indicate major or minor and any relevant stereochemistry. OH Br heat -8 IBr heat (c) Br NaCN DMFarrow_forward3. For the following reactions A-C, please draw the products including stereochemistry where appropriate. | a) H3C. сно b) A H3C H3C COOH c) COOHarrow_forward
- Please show the major products with stereochemistry where needed.arrow_forwardThe following are several isomers of C10H14 with two fused six-membered rings. A B D E G H (a) Identify which will react with ethene in a Diels-Alder reaction and which will not. (b) Draw one more isomer with two fused six-membered rings that will react with ethene in a Diels-Alder reaction.arrow_forwardProvide the structure of the product(s) with stereochemisty if appropriate. If multiple products indicate major product or if products are formed in equal amounts. If there is no reaction expected explain why. 1. H3180* 2. K2CO3 Mel CH3 1. CrO3, H30* H3C HO, 2. DCC, HN A, [-H2O] HOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY