
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't used hand raiting
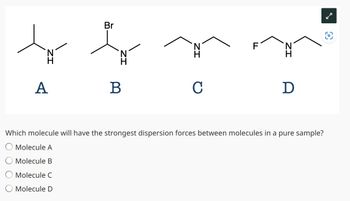
Transcribed Image Text:Br
N
H
IZ
A
B
C
D
Which molecule will have the strongest dispersion forces between molecules in a pure sample?
Molecule A
Molecule B
○ Molecule C
O Molecule D
8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 8.36 Why are dispersion forces attractive?arrow_forwardWhich of the following compounds would you expect to show dispersion forces? Dipole forces? (a) F2(b) CO (c) CO2 (d) H2COarrow_forwardIdentify the kinds of intermolecular forces (London dispersion, dipole-dipole, hydrogen bonding) that are important in each of the following substances. (a) propane (C3H8) (b) ethylene glycol [HO(CH2)2OH] (c) cyclohexane (C6H12) (d) phosphine oxide (PH3O) (e) nitrogen monoxide (NO) (f) hydroxylamine (NH2OH)arrow_forward
- The London dispersion forces are present in O CGH12 O HCI O H20 O All of the abovearrow_forwardWhich compound will have the strongest dispersion forces? O CH3F O NH3 SBr5 Ar O C4H10arrow_forwardBased on the Lewis structures shown, how many of the substances shown would experience dispersion forces with another molecule of the same structure? H. Н H-F: :CI-Be -CI: Н-С— H- H. H. hydrofluoric acid beryllium chloride dimethyl etherarrow_forward
- Which type(s) of molecular forces are present in the compound SF2?arrow_forwardThe very high melting point and boiling point of ionic compounds such as NaCl is due to the very strong intermolecular forces that exist between the particles. O Dispersion O Hydrogen bonding O Dipole-Dipole O Coloumbicarrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name chloromethane methanimine compound N,N- dichloromethylamine formula or Lewis structure CH₂Cl H H-C=N-H H:CI: | H-C- H CI: Between molecules of the compound? 00 yes no yes no yes hydrogen-bonding force no Between molecules of the compound and molecules of water? 00 00 yes no yes no yes no Xarrow_forward
- 1. This question will compare the three molecules, A - C shown below. Your task is to evaluate the intermolecular forces that exist in pure samples of each compound and in solutions to answer the questions about the physical properties below. A В H H CEN: :N-CH C=P H H C2H3N CH;N CH3Parrow_forwardPlease help answer this questionsarrow_forwardWhich one of these molecules has dipole-dipole forces? O CBr4 O OF2 O CH3CH2CH2CH3 O CS2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning