
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
please answer all parts!
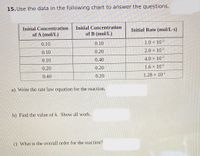
Transcribed Image Text:15. Use the data in the following chart to answer the questions.
Initial Concentration
Initial Concentration
Initial Rate (mol/L·s)
of A (mol/L)
of B (mol/L)
0.10
0.10
1.0 × 103
0.10
0.20
2.0 x 103
0.10
0.40
4.0 x 103
0.20
0.20
1.6 x 102
0.40
0.20
1.28 x 101
a) Write the rate law equation for the reaction.
b) Find the value of k. Show all work.
c) What is the overall order for the reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The label on a bottle of 3% (by volume) hydrogen peroxide, H2O2, purchased at a grocery store, states that the solution should be stored in a cool, dark place. H2O2decomposes slowly over time, and the rate of decomposition increases with an increase in temperature and in the presence of light. However, the rate of decomposition increases dramatically if a small amount of powdered MnO- is added to the solution. The decomposition products are H2O and O2. MnO2 is not consumed in the reaction. Write the equation for the decomposition of H2O2. What role does MnO2 play? In the chemistry lab, a student substituted a chunk of MnO2 for the powdered compound. The reaction rate was not appreciably increased. WTiat is one possible explanation for this observation? Is MnO2 part of the stoichiometry of the decomposition of H2O2?arrow_forwardSubstances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons before they reach the catalyst. Among the commonly encountered catalyst poisons are silicon and phosphorus, which typically form phosphate or silicate ions in the oxidizing environment of an engine. Group 2 elements are added to the catalyst to react with these contaminants before they reach the working portion of the catalytic converter. If estimates show that a catalytic converter will be exposed to 625 g of silicon during its lifetime, what mass of beryllium would need to be included in the design?arrow_forwardThe following equation represents a reversible decomposition: CaCO3(s)CaO(s)+CO2(g) Under what conditions will decomposition in a closed container proceed to completion so that no CaCO3 remains?arrow_forward
- Determine rxnH 25 C for the following reaction: NO g O2 g NO2 g This reaction is a major participant in the formation of smog.arrow_forwardExplain how a species might be part of a rate law but not part of a balanced chemical reaction.arrow_forwardDistinguish between the differential rate law and the integrated rate law. Which of these is often called just the rate law? What is k in a rate law, and what are orders in a rate law? Explain.arrow_forward
- . Account for the increase in reaction rate brought about by a catalyst.arrow_forwardCandle wax is a mixture of hydrocarbons. In the reaction of oxygen with candle w ax in Figure 11.2, the rate of consumption of oxygen decreased with time after the flask was covered, and eventually' the flame went out. From the perspective of the kinetic-molecular theory, describe what is happening in the flask. FIGURE 11.2 When a candle burns in a closed container, the flame will diminish and eventually go out. As the amount of oxygen present decreases, the rate of combustion will also decrease. Eventually, the rate of combustion is no longer sufficient to sustain the flame even though there is still some oxygen present in the vessel.arrow_forwardA reaction of the form aAProducts gives a plot of ln[A] versus time (in seconds), which is a straight line with a slope of 7.35 X 103. Assuming [A]0 = 0.0100 M, calculate the time (in seconds) required for the reaction to reach 22.9% completion.arrow_forward
- One experimental procedure that can be used to determine the rate law of a reaction is the method of initial rates. What data are gathered in the method of initial rates, and how are these data manipulated to determine k and the orders of the species in the rate law? Are the units for k. the rate constant, the same for all rate laws? Explain. If a reaction is first order in A, what happens to the rate if [A] is tripled? If the initial rate for a reaction increases by a factor of 16 when [A] is quadrupled, what is the order of n? If a reaction is third order in A and [A] is doubled, what happens to the initial rate? If a reaction is zero order, what effect does [A] have on the initial rate of a reaction?arrow_forwardAmoxicillin is an antibiotic packaged as a powder. When it is used to treat babies and small animals, the pharmacist or veterinarian must suspend it in water, so that it can be administered orally with a medicine dropper. The label says to dispose of unused suspension after 14 days. It also points out that refrigeration is required. In the context of this chapter, what is implied in the latter two statements?arrow_forwardOne of the concerns about the use of Freons is that they will migrate to the upper atmosphere, where chlorine atoms can be generated by the following reaction: CCl2F2(g)Freon-12hvCF2Cl(g)+Cl(g) Chlorine atoms can act as a catalyst for the destruction of ozone. The activation energy for the reaction Cl(g) + O3(g) ClO(g) + O2(g) Is 2.1 kJ/mol. Which is the more effective catalyst for the destruction of ozone, Cl or NO? (See Exercise 75.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax