
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
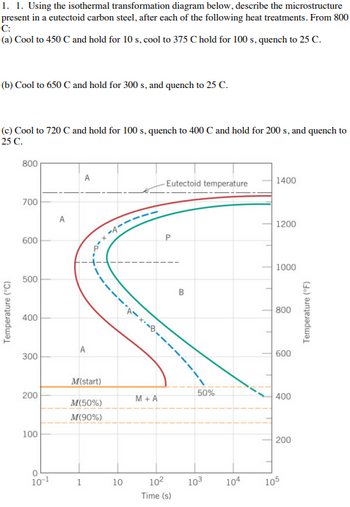
Transcribed Image Text:1. 1. Using the isothermal transformation diagram below, describe the microstructure
present in a eutectoid carbon steel, after each of the following heat treatments. From 800
C:
(a) Cool to 450 C and hold for 10 s, cool to 375 C hold for 100 s, quench to 25 C.
(b) Cool to 650 C and hold for 300 s, and quench to 25 C.
(c) Cool to 720 C and hold for 100 s, quench to 400 C and hold for 200 s, and quench to
25 C.
Temperature (°C)
800
700
600
500
400
300
200
100
0
10-1
A
A
A
M(start)
M(50%)
M(90%)
1
10
M+A
Eutectoid temperature
P
10²
Time (s)
B
50%
10³ 104
1400
1200
1000
800
600
400
200
105
Temperature (°F)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Consider an AISI 1045 ferrite and pearlite microstructure. The sample is initially heated to 845 deg C and quenched in water. The samples are then tempered for 20 minutes at 316 deg c, 425 deg c, and 600 deg c. Can yu tell me what would happen to the microstructure hardness os the samples as a result of quenching, tempering theory wise.arrow_forwardB- Describe the microstructure and mechanical properties produced in carbon steel which contain 0.5wt% carbon ,when subject to the following heat treatment:- 1- Heated to 870°C and water quenched to room temperature. 2- Heated to 870°C and furnace cooled to room temperature. 3- Heated to 870°C and cooled in air to room temperature.arrow_forwardPlease draw each scenario using the TTT diagramarrow_forward
- Of the following alloys, pick the one(s) thatmay be strengthened by heat treatment,cold work, or both: R50250 titanium, AZ31Bmagnesium, 6061 aluminum, C51000 phos-phor bronze, lead, 6150 steel, 304 stainlesssteel, and C17200 beryllium copper.arrow_forwardplease draw each question on the TTT diagramarrow_forwardThe Iron-Carbon system: Phase Diagram 1. You heat treated a 1045 steel plate by heating it to 900°C and slowly cooling it to 725 °C, which is below the eutectoid temperature. What phases are present at 725°C? What are the compositions of each phase at 725°C? What are the weight fractions of each phase at 725°C? What microconstituents are present at 725°C? What are the compositions of each microconstituent at 725°C? What are the weight fractions of each microconstituent at 725°C?arrow_forward
- Do not give answer in image and hand writingarrow_forward7. Consider 1.5 kg of a 99.6 wt% Fe-0.4 wt% C steel that is cooled to a temperature just below the eutectoid. (a) How many kilograms of ferrite (a) form? (b) How many kilograms of cementite (Fe;C) form? Include your tie line in the solution. 1600| L 1400 T(°C) 1200 (austenite) y+L 1148°C L+Fe;C 1000 Y + Fe;C 800 727°C 600 a + Fe;C 400 1 2 4 6 6.7 C, wt% C Fe;C (cementite)arrow_forwarda: Quench to from 800oC to 350oC and hold for 102 seconds:b: Quench the alloy to room temperaturec: Reheat the alloy to 700oC and hold for 105 secondsarrow_forward
- Using the time-temperature-transformation diagram, what steel phases would you generate by cooling from 900ºC A. quenching in cold water to 520ºC in 0.5 second, holding the sample temperature constant for 1000 seconds at 520ºC, and then quenching to room temperature B. cooling down to room temperature over a period of 2 weeks?arrow_forwardA.2 (i) (ii) Explain how an understanding of the creep failure mechanisms of high temperature nickel alloys has led to alloy and manufacturing developments which have resulted in an increase in the maximum operating temperature of a jet engine turbine blade. If we quench a plain carbon steel we can produce a very strong/hard and yet brittle material. Why does the quench lead to this change in properties, and what subsequent heat treatment would enable us to mostly retain the high strength whilst improving the ductility? Explain the effects of your proposed heat treatments in terms of the phase changes and microstructural features developed at each stage.arrow_forwardLook up the standard aluminum alloy heat treatment temper designations. Use the designations to justify the yield strength behavior of the following aluminum alloys listed in Table 1.2: 2024-T3 vs. 2024-T6 and 7075-T6 vs. 7075-T73.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY